As we start to see treatments emerge for dementia, we expect the effects to be quite modest at first. It would typically take further years or decades of therapeutic development to bring larger benefits and something more resembling a 'cure'. However, with recent breakthroughs in genetic technology, a more permanent solution may not seem so far off for some neurodegenerative diseases. To learn more, we spoke to Prof Vincent Dion, Group Leader from UK DRI at Cardiff and DNA repair theme Lead, whose work into gene editing is at the cutting-edge of the search for therapeutics.
Huntington’s disease is a devastating neurodegenerative condition which often tragically impacts people in their forties, causing abnormal movements and the gradual inability to think and communicate before death around 15 years later. Unlike a more common disease associated with dementia such as Alzheimer’s, Huntington’s has a purely genetic origin, with an individual having a 50% chance of inheriting the condition if a parent is affected.
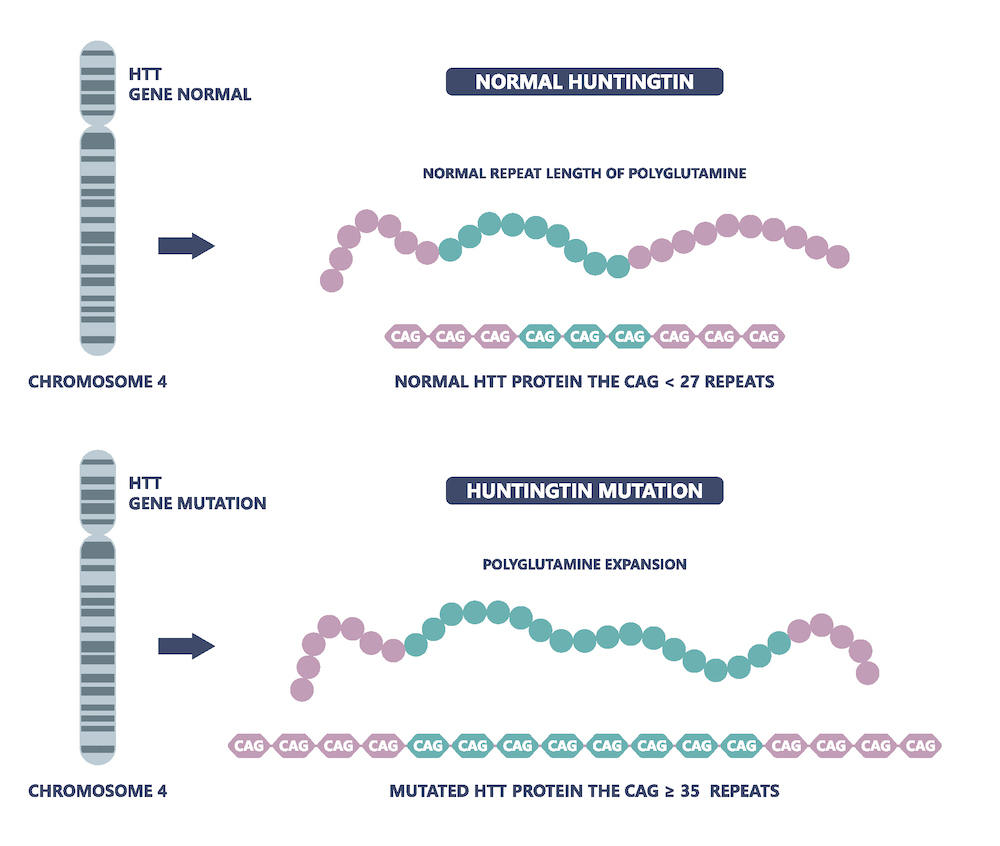
The underlying biological cause of Huntington’s, and at least 14 other neurological and neuromuscular diseases, is an increased or expanded number of repetitive stretches of DNA, for example the sequence CAG/CTG (CAG repeats). The resulting Huntington’s protein is toxic and thought to have many harmful effects in the brain including interference with a neuron’s transport network and gene regulation, leading to the clinical symptoms observed. Additionally, the more CAG repeats that a person has, the more severe the manifestation of the disease.