Correcting faulty genes in neurodegenerative disease
Amyotrophic lateral sclerosis (ALS, the most common form of motor neurone disease (MND)) and frontotemporal dementia (FTD) are two examples of devastating neurodegenerative disease. While not all symptoms are shared between the two diseases (ALS primarily affecting movement, FTD primarily affecting behaviour and personality), there is significant overlap in their genetic causes and underlying biology.
The Shaw Lab are studying FTD and ALS to advance our understanding of their molecular causes and to develop effective new targeted treatments.
A key goal is to develop an innovative new gene therapy for people with a type of FTD caused by faults in a specific gene. The approach involves delivering the correct copy of the gene into their body. In 2021, Prof Shaw launched a UK DRI spin-out from King's College London, Aviadobio, focusing on gene therapy as an approach to treat neurodegenerative diseases, based on the principle of delivering DNA into cells to supplement or knock down mutated genes.
In April 2024, AviadoBio announced they had treated their first patient in ASPIRE-FTD clinical trial evaluating AVB-101 for frontotemporal dementia with GRN mutations.
Latest news

Prof Chris Shaw
Prof Chris Shaw is a Group Leader at the UK DRI at King's. Find out more about his career and expertise on his profile page.

Research summary
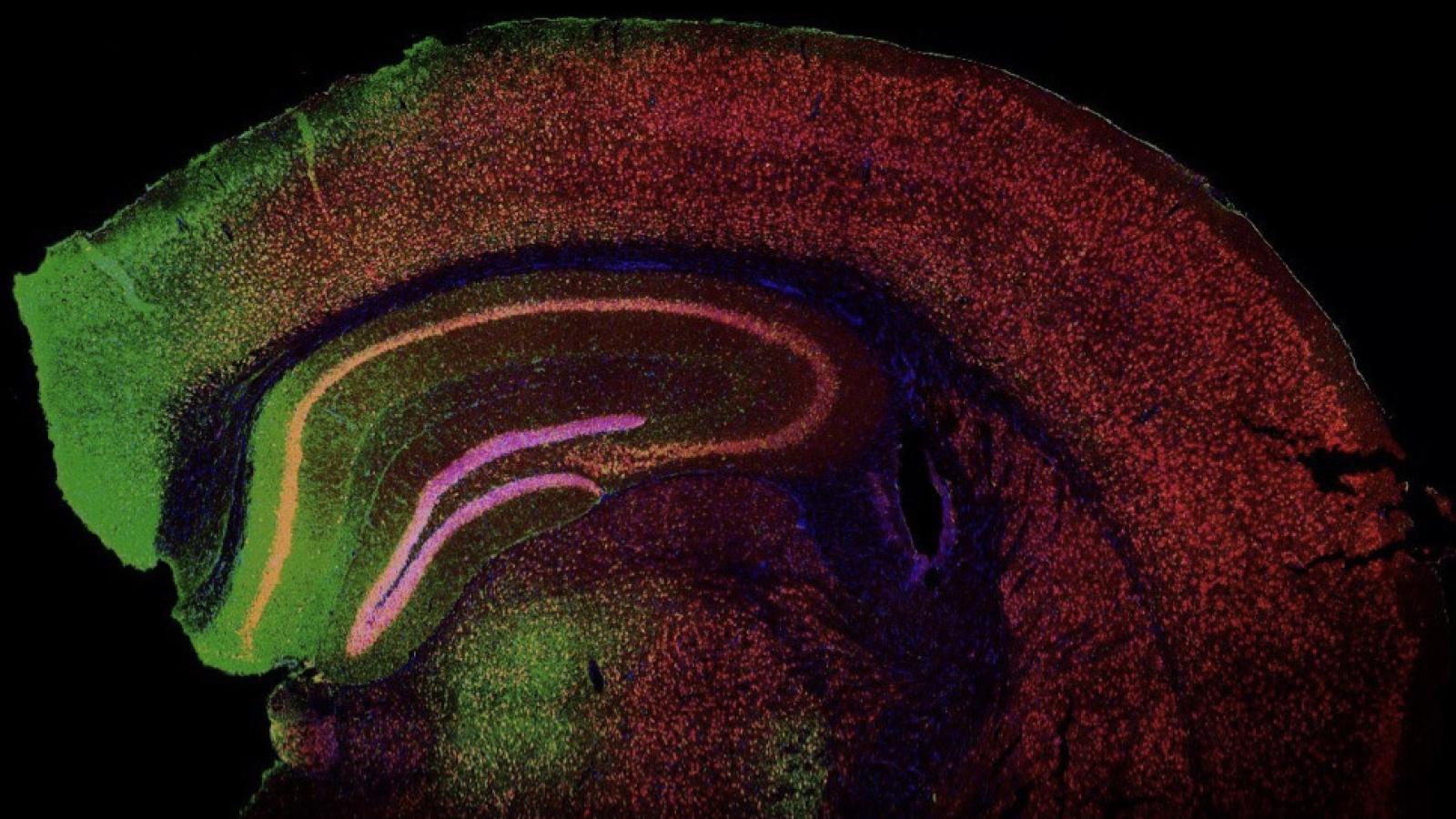
Image of green fluorescent protein (GFP) expression in the mouse brain cortex following adeno-associated viral (AAV) vector injection.
Credit: Shaw Lab.
Proteostatic mechanisms in FTD and ALS
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are two closely related neurodegenerative conditions with significant overlap in clinical, pathological, radiological, and genetic characteristics; an intronic GGGGCC repeat expansion in C9orf72 is the most common genetic cause of both disorders. The Shaw Lab are studying FTD and ALS to advance our understanding of their molecular causes and to develop effective new targeted treatments.
The Shaw Lab are initiating a new gene therapy programme as part of Chris's UK DRI programme, based on the development of adeno-associated viral (AAV) vectors capable of delivering safe, long-lasting and cost-effective gene therapies for neurodegenerative disorders in appropriately selected patient populations. Although several trials of intrathecal antisense oligonucleotides therapies in a range of neurodegenerative disorders are currently underway, no trials have yet been conducted using AAV vectors. Thus, crucial preclinical experiments could pave the way to first-in-human experimental medicine studies with the potential to carve a path for the development of many more AAV vector delivered therapies in neurodegeneration.
Alongside the development of novel gene therapy approaches, the team is also investigating TDP-43 aggregates, which are the hallmark pathology in 95% of all ALS and tau-negative FTD cases. TDP-43 is a DNA and RNA binding protein that regulates RNA transcription, splicing, trafficking and translation. The group plan to map the earliest molecular events that promote TDP-43 mislocalisation and aggregation as well as endogenous proteostatic neuroprotective response in patient iPSC-derived neurons carrying a range of proteostatic gene defects, and TDP-43 transgenic mice. Key degenerative and protective signatures will be validated in human post-mortem tissues. Armed with a greater understanding of the factors that promote TDP-43 aggregation and regulate its clearance, they will seek to manipulate these pathways using gene knockdown/overexpression and small molecules in order to develop a rational therapeutic strategy aimed at preventing aggregation and enhancing endogenous proteostasis.
Main objectives and research goals:
- To develop novel AAV-based gene therapy therapeutic approaches. Optimising AAV9 vectors for gene delivery into the CNS from preclinical experiments in cells and rodents up to first-in-human experimental medicine studies.
- To map TDP-43 proteostasis in vitro using human iPSc-derived neurons: Phenotypic analyses including transcriptomics, proteomics and high-content imaging in cultured motor neurons – including patient-derived iPSC and isogenic lines generated by genome editing with CRISPR/Cas9.
Key publications
Vacancies
Lab members
- Dr Neda Ali Mohammadi Nafchi (Postdoctoral Researcher)
- Dr James Bashford (Postdoctoral Researcher)
- Dr Bibiana Mota (Postdoctoral Researcher)
- Dr Andrea Perera (Postdoctoral Researcher)
- Dr Gillian Kelly (Postdoctoral Researcher)
- Dr Neda Ali Mohammadi Nafchi (Postdoctoral Researcher)
- Dr Lucia Dutan-Polit (Postdoctoral Researcher)
- Yalem Bekele (Senior Senior Vector Viral Scientist)
- Emma Salmela (Research Assistant)
- Miryam Ravji (Research Assistant)
- Meagan Chamberlain (Research Assistant)
- Quwam Akiniawon (Technician)
- Sally McPherson (Laboratory Technician)
- Joshua Yeung (Technician)
- Malgorzata Bien-Novakowska (Technician)
- Afra Aabadien (PhD Student)
- Judith Bilgorai (PhD Student)
Collaborators
Lab funders
Thank you to all those who support the Shaw Lab!