

UK DRI at Imperial

The UK DRI at Imperial brings new perspectives to prevent, delay or reverse dementia. We focus on the earliest stages when benefits to those who may be affected will be greatest.

Contact
Located at Imperial’s White City campus, the UK DRI at Imperial brings together researchers from diverse backgrounds with fresh perspectives.
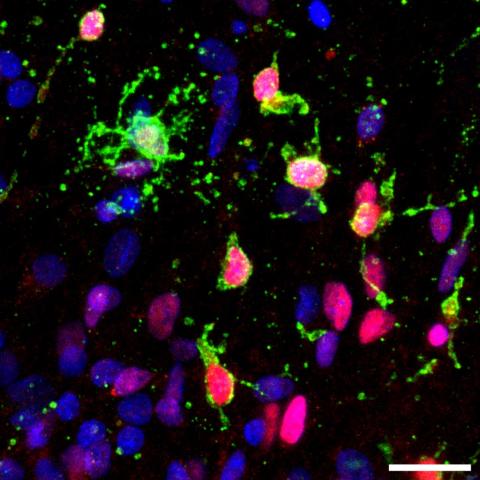
The team recognises that the challenges of dementia demand new concepts, new approaches and a diverse range of research tools and directions. Their holistic approach views the ageing brain, not in isolation, but in the context of the ageing body. Researchers are studying individual cells through to whole systems, to unveil new understanding of what can go wrong in the brain and lead to dementia.
The team’s multidisciplinary approach brings new expertise to the challenges of dementia research. By exploring individual variation across populations, influences of the gut on the brain, mutual interactions between neurons and support cells in the brain, and the role of sleep, researchers are addressing exactly how vulnerable cells and systems go on to develop neurodegenerative diseases.
Latest news


People
Scientific focus
Read about the scientific focus of the UK DRI at Imperial












