Huntington's is a neurodegenerative disease that has purely genetic origins, and devastating consequences. In the search for a possible solution for people who are affected by this condition, researcher Prof Vincent Dion, from the UK DRI at Cardiff, has developed an innovative gene editing technique, harnessing technology based on CRISPR to correct the mutation and halt the progression of the disease.
This article is part of our ‘Discover’ series, framed by the UK DRI's mission to discover the causes of neurodegeneration, develop possible treatments and deliver solutions for healthy ageing.
Within our DNA there are sections of code that repeat. For example, humans typically have up to 34 CAG/CTG repeated sequences in the Huntingtin gene, which is linked to Huntington’s disease. However, due to hereditary causes, this number may be higher in some people. When the CAG or CTG triplet is repeated more than 35 times, a person may be affected by Huntington’s. There are at least 15 diseases caused by repeated sequences like this, and they are known as repeat expansion diseases.
The Dion Lab has been working for some time to develop an innovative treatment capable of reducing the repetitions of these triplets, and thus preventing the progression of Huntington's.
Curiosity seeding solutions
It was a dissected rat that caught Dion's attention at the age of 11, when his school went on a visit to a high school. It wasn't until three years later that it was his turn to study biology, but once he did, that sparked his decision to pursue science.
During his PhD studies at Baylor College of Medicine (USA), Vincent became interested in understanding more about the causes of repeat expansion diseases and how to target them.
“We found out that we could actually manipulate the size of the repeat, which is the cause of Huntington’s and a number of related disorders. We found this out completely by chance, so, basically, my curiosity about DNA repair got me there,” Vincent says.
In science you are told to "follow the results". By trying to decipher certain mysteries of how things work, fascinating discoveries can be made that lead to the development of new techniques or treatments. For example, CRISPR, the gene-editing technology on which Vincent's work is based, arose from the study of the immune system of a certain bacterium; eventually the results would lead scientists to transfer what they had discovered in this area to an instrument capable of editing DNA.
“When I started my lab, we found out completely by chance that you could shrink the repeats back down. I found myself going from a very basic question about mechanistic molecular detail to looking at a potential way to have a very effective treatment for these neurodegenerative diseases,” he adds.
Innovation to predict, diagnose and treat
But why does the CAG or CTG sequence expansion pose a problem for people who have it in their DNA? The protein that ends up forming from this mutation is toxic. It is thought to have many harmful effects on the brain, including interference with the neurons' transport network and gene regulation. Together, the toxic effects lead to the death of neurons and ultimately abnormal movements, the gradual inability to think and communicate, and an early death.
“It turns out that this abnormal protein is sticky, so it binds to a lot of other things and takes them away from what they are supposed to be doing. This is why it is said that the protein is toxic,” explains the investigator.
In the search for a possible solution, the Dion Lab developed an algorithm that precisely counts the number of DNA repeats present for a person affected by Huntington's or other repeat expansion disorders. This is important because it forms the basis of a diagnostic test: the higher the number of DNA repeats, the more likely it is that the disease will appear at an earlier age. The algorithm can also detect if there are disruptions in the sequence which may help to predict when someone will begin experiencing symptoms.
“The interruptions within the repeat tend to delay disease onset, so knowing not just the size, but whether it's peppered with other sequences, will give a better idea of when to expect someone to develop the disease,” Vincent says.
Researchers at the UK DRI at Cardiff have worked not only to improve diagnosis, but also to find a way to target these DNA repeats. It is a technique that, unlike that proposed by CRISPR-Cas9 technology, does not involve editing both strands of the DNA molecule, but only one to induce contractions in the repeated sections of DNA exclusively, without mutating the flanking sequences. To achieve this, the team has developed a mutated CRISPR-Cas9 enzyme known as a nickase.
By shrinking the repeat track, we correct the mutation and so we remove the cause of the disease. We might be able to stop the disease from progressing any further or if the treatment is given at an early stage we can hope to prevent it from appearing in the first place.Prof Vincent Dion
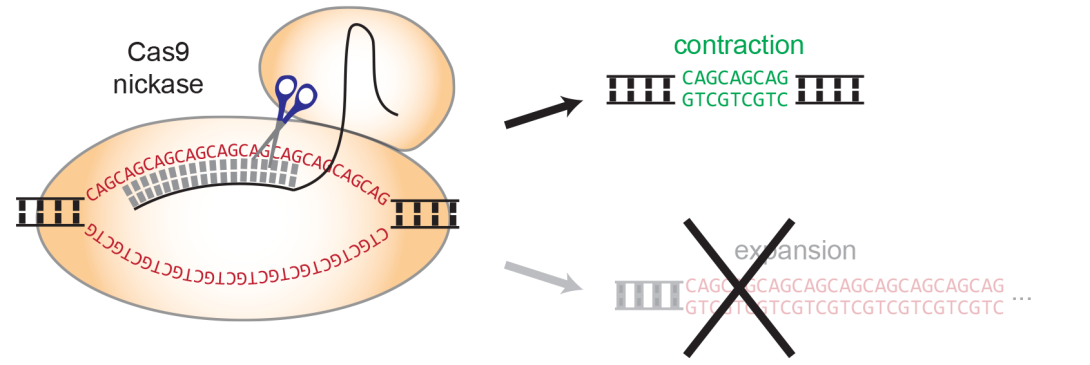
developed by Vincent.
From the lab to the clinic
Since their emergence in the early 21st century, gene-editing tools such as CRISPR have been under scrutiny for their ethical implications. Some studies indicate that gene-editing techniques can produce off-target genome editing effects, or even cancer, if rogue rearrangements are generated in the DNA. Vincent and his team are working hard to understand more about those risks, so potential future treatments can be as safe as possible.
Today, clinical trials are already underway in blood, liver and eye diseases, as the procedures are simpler and, in some cases, the consequences are reversible. Results from the Dion Lab obtained so far are also moving ever closer to testing the gene-editing technique in humans.
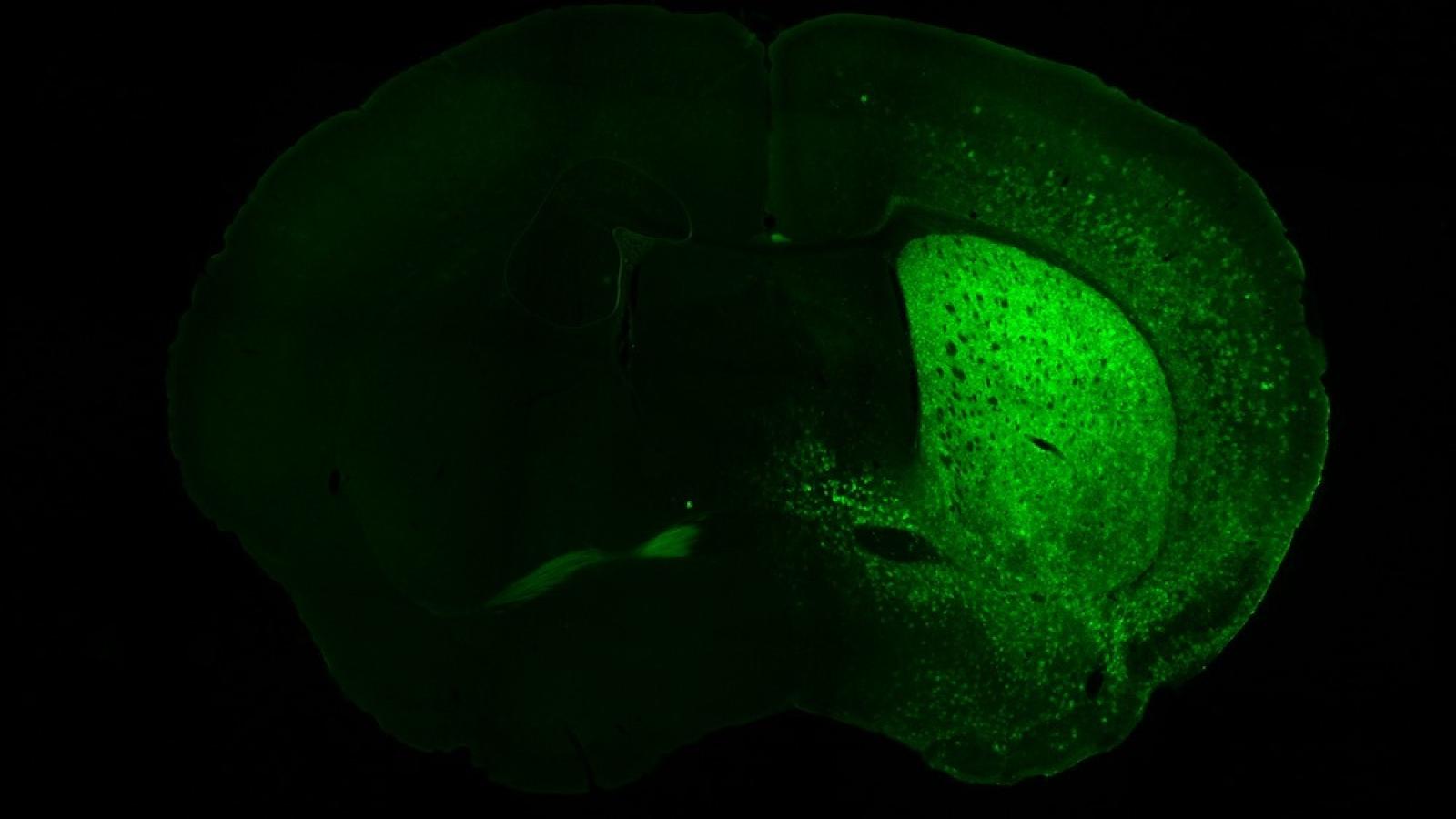
What started with human cells in a dish, to understand whether the technique could be performed as proof of concept, has evolved to trialling in animal models utilising a harmless viral system to deliver a molecular package to affected neurons in a mouse model of Huntington’s disease: testing the technique in a living organism has enabled the researchers to understand whether symptoms improved when the mutation was corrected.
“It works well in mice. That suggests that we should be able to do it in any living organism, but we don’t know for sure, so that is what we are doing next: we will try it in rats. If this works in multiple different model organisms, then we have a better chance of having it working well in people. Our highest hopes are to stop the progression of the disease or even prevent it from appearing in the first place,” Vincent concludes.
Article published: 9 September 2024
Image credits: Prof Vincent Dion