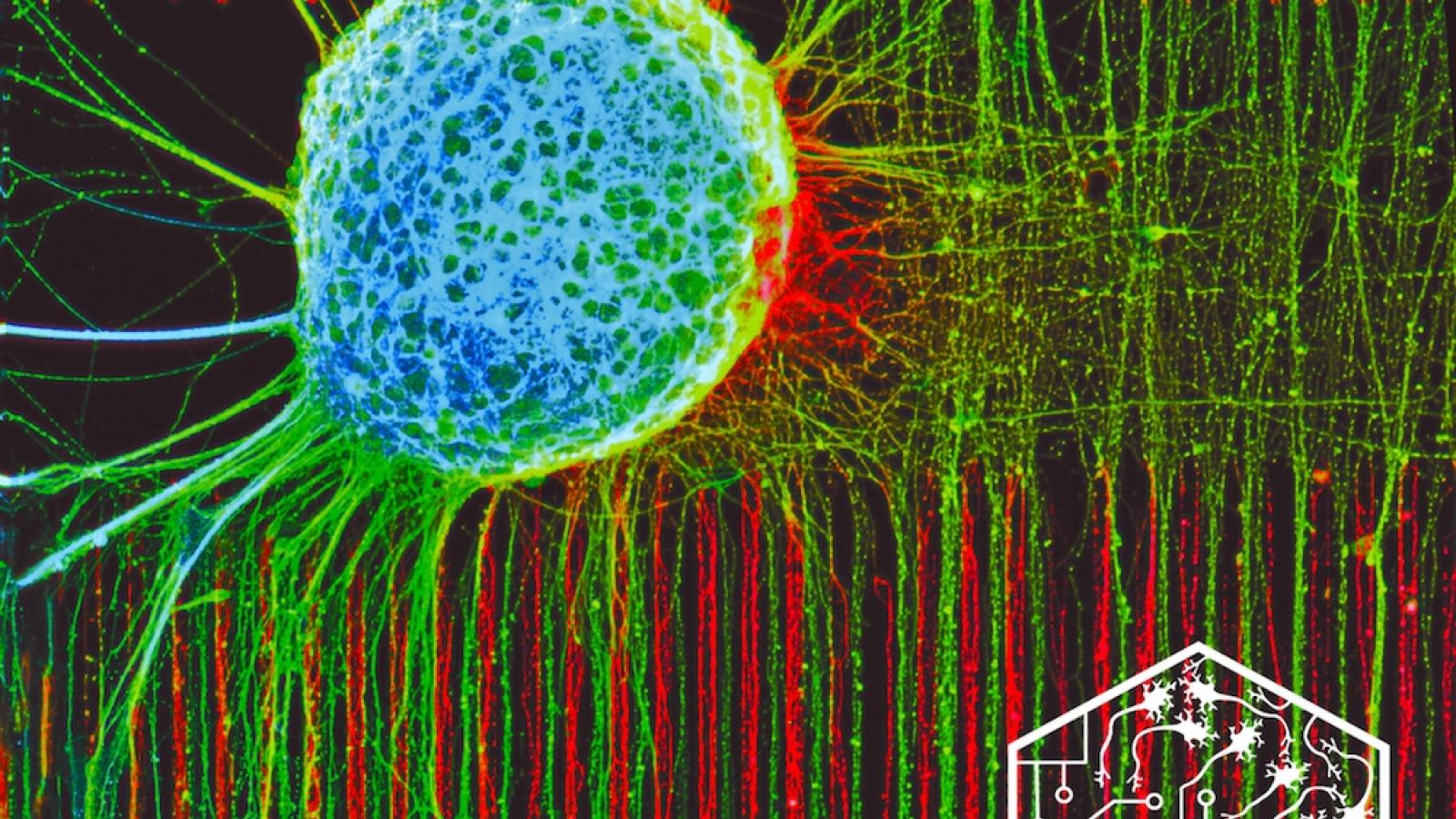
To fully understand the complex processes and components that make up our nervous system and how they go wrong in conditions like motor neuron disease (MND), we need to be able to build model systems that closely reflect the processes that occur within the human body.
Dr Andrea Serio, the UK DRI’s newest Group Leader, joins the UK DRI at King’s where he will be tackling MND from a unique angle. By applying principals from his background in bioengineering, he aims to build better systems to model the disease. Using engineering and physics to study the interplay between the shape and structure of a cell and its biology, he hopes to gain a better understanding of the fundamental mechanisms involved in disease. Below, Dr Serio discusses his new UK DRI programme and where he hopes his work will lead.
What attracted you to the UK DRI?
For the past 5 years, my lab has been developing tools and technologies to probe a bit better into human neurobiology. We’ve been tackling questions related to how neurons and circuits are engineered, physically constructed and how they work in the real world. I’ve had a long-standing interest in disease modelling; I started my PhD a long time ago modelling amyotrophic lateral sclerosis (ALS) in vitro, and trying to explain neurodegeneration with human models.
My lab joining the UK DRI at King’s happened as a result of a nice combination of our work reaching a point of maturity and the Centre going in a new direction with Jernej Ule as its new Director. I was already working with Jernej on other projects, and we got talking and realised it would be the perfect time to apply some of the discoveries my lab has made, directly to neurodegeneration and MND/ALS. So it just made sense for us, we’re following the direction of the science. I’m really excited about the opportunity, it’s an amazing centre to be in and we’ll be working alongside an excellent group of colleagues.
What will your research programme focus on?
I am an engineer, and a biotechnologist by training so I have a very practical mind. As I have moved through my biomedical career, I’ve often wondered about how things are shaped and why we don’t pay more attention to the engineering and the physics of it.
Our recent discoveries show that cells are built in a specific way for a reason. Neurons have a long extension, an axon, that connects different parts of body that are sometimes metres away. There is specific interplay between physics and biology in the neuron, and the molecular mechanisms that are involved in disease. This made us realise there is a potential to look at models of MND in vitro in a completely different way and extract more meaning from them, in order to better understand the biology.
Specifically, we’ll be looking at the axonal biology of motor neurons – how the length, size and shape influences the RNA and mitochondrial biology within the axon. Through this, we think we might find some of the early mechanisms by which the neuron dies in ALS/MND. At the same time we are collaborating with a number of people within the UK DRI to bring the modelling platforms, circuit building and bioengineering systems we’ve already developed directly to disease modelling and potentially even to the clinic in some of the programmes that involve screening for drugs.

Why is this an important area to explore? And what is the ultimate aim of your work?
I think the interface between neurobiology, and physics and engineering has been neglected historically. Cells and circuits are real objects that interact with the world and are often constrained by shape, length, size of targets. We sometimes overlook that in pursuit of biological mechanisms. We think it’s important to take these things into account. By taking control of the engineering of cells, we can first of all build better models – and this means we can gain a better understanding of how these cells work which translates to a much better angle on the disease.
What are you particularly excited about in your field at the moment?
The concept of integrating synthetic biology in neurodegeneration. Using synthetic biology to allow us to take control of mutant protein expression, localisation, downstream effect or even just the starting and stopping of certain disease processes. I think this is going to be ultimately what closes the circle on having full control of a system and allows us to make some really interesting discoveries.
Finally, what’s the most important thing you have learned from your academic career so far?
Fail often, and learn from it. I think there’s sometimes a bit too much emphasis on successes in academia. That’s all well and good but it creates a fairly warped vision of what actually happens. Everyone is here because they had a past career, therefore everyone has made mistakes. More often than not focusing on only the good things isn’t realistic. There needs to be more recognition that failure is the most important teacher. We need to try things and fail, and learn from them quickly, which is what ultimately leads to success.
Links
· Dr Serio’s UK DRI profile
· Q&A with Group Leader Dr Sarah Marzi
· Feature with Centre Director Prof Jernej Ule
Article published: 24 July 2023
Banner image credit: Dr Andrea Serio