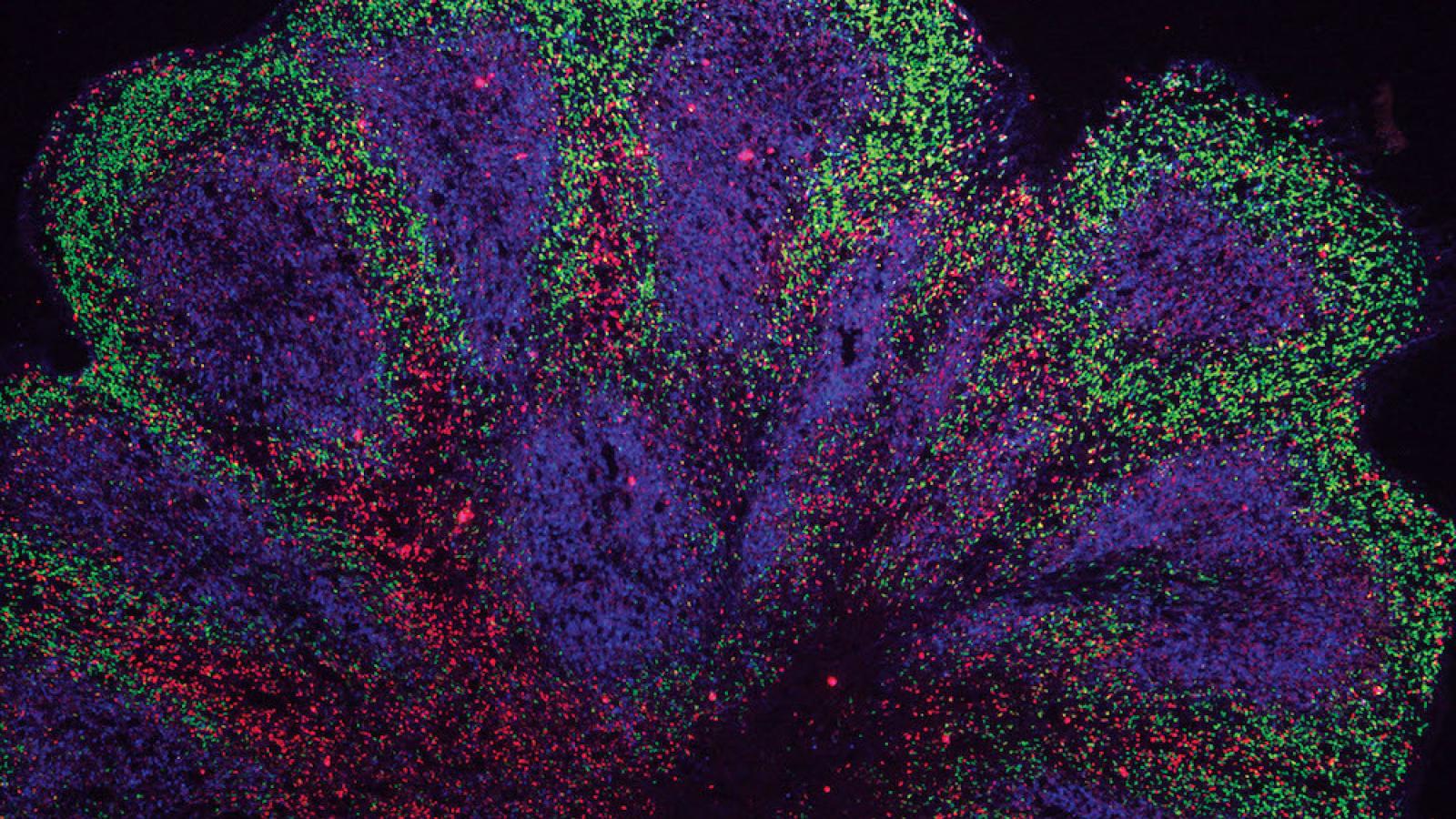
Researchers from the University of Cambridge, including UK DRI Group Leader Dr Gabriel Balmus, have investigated the earliest stages of the neurological conditions amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). They used a novel human organoid (mini brain) model developed in the laboratory of Dr Andras Lakatos (Department of Clinical Neurosciences), which may help to better understand the underlying disease biology and will serve as a powerful tool for screening drugs to prevent or slow disease progression in the future.
A common form of motor neurone disease, amyotrophic lateral sclerosis, often overlaps with frontotemporal dementia (ALS/FTD) and can affect younger people occurring mostly after the age of 40-45. These conditions cause devastating symptoms of muscle weakness with changes in memory, behaviour and personality. In order to decipher disease mechanisms and find potential targets for treatments, researchers are increasing using innovative cellular models, such as brain organoids, to study human biology and disease.
Typically when developing organoids, researchers take cells from a patient’s skin and reprogramme the cells back to their stem cell stage – a very early stage of development at which they have the potential to develop into most types of cell. These can then be grown in culture as 3D clusters that mimic particular elements of an organ. As many diseases are caused in part by defects in our DNA, this technique allows researchers to see how cellular changes – often associated with these genetic mutations – lead to disease. At the University of Cambridge alone, researchers use them to repair damaged livers, study SARS-CoV-2 infection of the lungs and model the early stages of pregnancy, among many other areas of research.
In the groundbreaking study published today in Nature Neuroscience, scientists at the John van Geest Centre for Brain Repair, University of Cambridge, used stem cells derived from people living with ALS/FTD to grow brain organoids. These resemble parts of the human cerebral cortex in terms of their embryonic and fetal developmental milestones, 3D architecture, cell-type diversity and cell-cell interactions. Although this is not the first time scientists have grown mini brains from patients with neurodegenerative diseases, most efforts have only been able to grow them for a relatively short time frame, representing a limited spectrum of dementia-related disorders. The team report growing these models for 240 days from stem cells harbouring the commonest genetic mutation in ALS/FTD, which was not previously possible – and in unpublished work say they have grown them for 340 days.
The brain organoids were grown for 240 days from stem cells harbouring the commonest genetic mutation in ALS/FTD, which was not previously possible
We currently have no very effective options for treating ALS/FTD, and while there is much more work to be done following our discovery, it at least offers hope that it may in time be possible to prevent or to slow down the disease process.Dr András Lakatosthe senior author who led the research in Cambridge’s Department of Clinical Neurosciences
Dr András Lakatos, the senior author who led the research in Cambridge’s Department of Clinical Neurosciences, said:
“Neurodegenerative diseases are very complex disorders that can affect many different cell types and how these cells interact at different times as the diseases progresses.
To come close to capturing this complexity, we need models that are more long-lived and replicate the composition of those human brain cell populations in which disturbances typically occur, and this is what our approach offers. Not only can we see what may happen early on in the disease – long before a patient might experience any symptoms – but we can also begin to see how the disturbances change over time in each cell.”
While organoids are usually grown as balls of cells, first author Dr Kornélia Szebényi generated patient cell-derived organoid slice cultures in Dr Lakatos’ laboratory. This technique ensured that most cells within the model could receive the nutrients required to keep them alive.
Dr Szebényi said:
“When the cells are clustered in larger spheres, those cells at the core may not receive sufficient nutrition, which may explain why previous attempts to grow organoids long term from patients’ cells have been difficult.”
Using this approach, Dr Szebényi and colleagues observed changes occurring in the cells of the organoids at a very early stage, including cell stress, damage to DNA and changes in how the DNA is transcribed into proteins. These changes affected those nerve cells and other brain cells known as astroglia, which orchestrate muscle movements and mental abilities.
“Although these initial disturbances were subtle, we were surprised at just how early changes occurred in our human model of ALS/FTD,” added Dr Lakatos. “This and other recent studies suggest that the damage may begin to accrue as soon as we are born. We will need more research to understand if this is in fact the case, or whether this process is brought forward in organoids by the artificial conditions in the dish.”
As well as being useful for understanding disease development, organoids can be be used to screen potential drugs to prevent or slow disease progression. This is a crucial advantage of organoids, as animal models often do not show the typical disease-relevant changes and sampling the human brain for this research would be unfeasible. The team showed that a drug, GSK2606414, was effective at relieving common cellular problems in ALS/FTD, including the accumulation of toxic proteins, cell stress and the loss of nerve cells, hence blocking one of the pathways that contributes to disease. Similar drugs that are more suitable as medications and approved for human use are now being tested in clinical trials for neurodegenerative diseases.
Dr Gabriel Balmus, Group Leader from UK DRI at Cambridge, collaborating senior author, said:
“By modelling some of the mechanisms that lead to DNA damage in nerve cells and showing how these can lead to various cell dysfunctions, we may also be able to identify further potential drug targets."
Dr Lakatos added:
“We currently have no very effective options for treating ALS/FTD, and while there is much more work to be done following our discovery, it at least offers hope that it may in time be possible to prevent or to slow down the disease process.
It may also be possible in future to be able to take skin cells from a patient, reprogramme them to grow their ‘mini brain’ and test which unique combination of drugs best suits their disease.”
The study was primarily funded by the Medical Research Council UK, Wellcome Trust and the Evelyn Trust.
Reference
Article published: 21 October 2021