Led by an international team including UK DRI Centre Director Chris Shaw at Kings College London, a new risk gene for motor neuron disease (MND, also known as amyotrophic lateral sclerosis, ALS) has been identified.
MND is a fatal neurodegenerative disorder that causes progressive muscle paralysis. On their hunt for new MND-associated genes, the team sequenced the DNA from 751 patients who had the familial form of the disease and from 180 with sporadic MND. They found six different mutations in a protein called ‘Annexin A11’ in 13 people that were absent in 70,000 people without the MND.
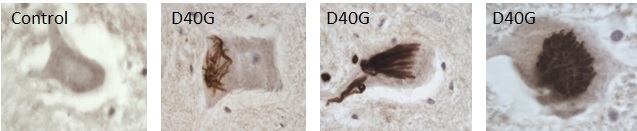
Fortunately, one patient carrying a mutation had donated their brain and spinal cord tissues to research. When the researchers looked at the distribution of Annexin A11 staining in this patient's motor neurons they found large tube-shaped aggregates which were not seen in any other cases.
Professor Shaw said, “We saw the most extraordinary pattern of aggregates when we looked under the microscope. This mutant Annexin A11 protein was actually aggregating in our patient's neurons. That gave me 100 percent confidence that we had found a real MND-causing gene.”
people had mutations in Annexin 11
We saw the most extraordinary pattern of aggregates when we looked under the microscope.Professor Chris ShawUK DRI Centre Director
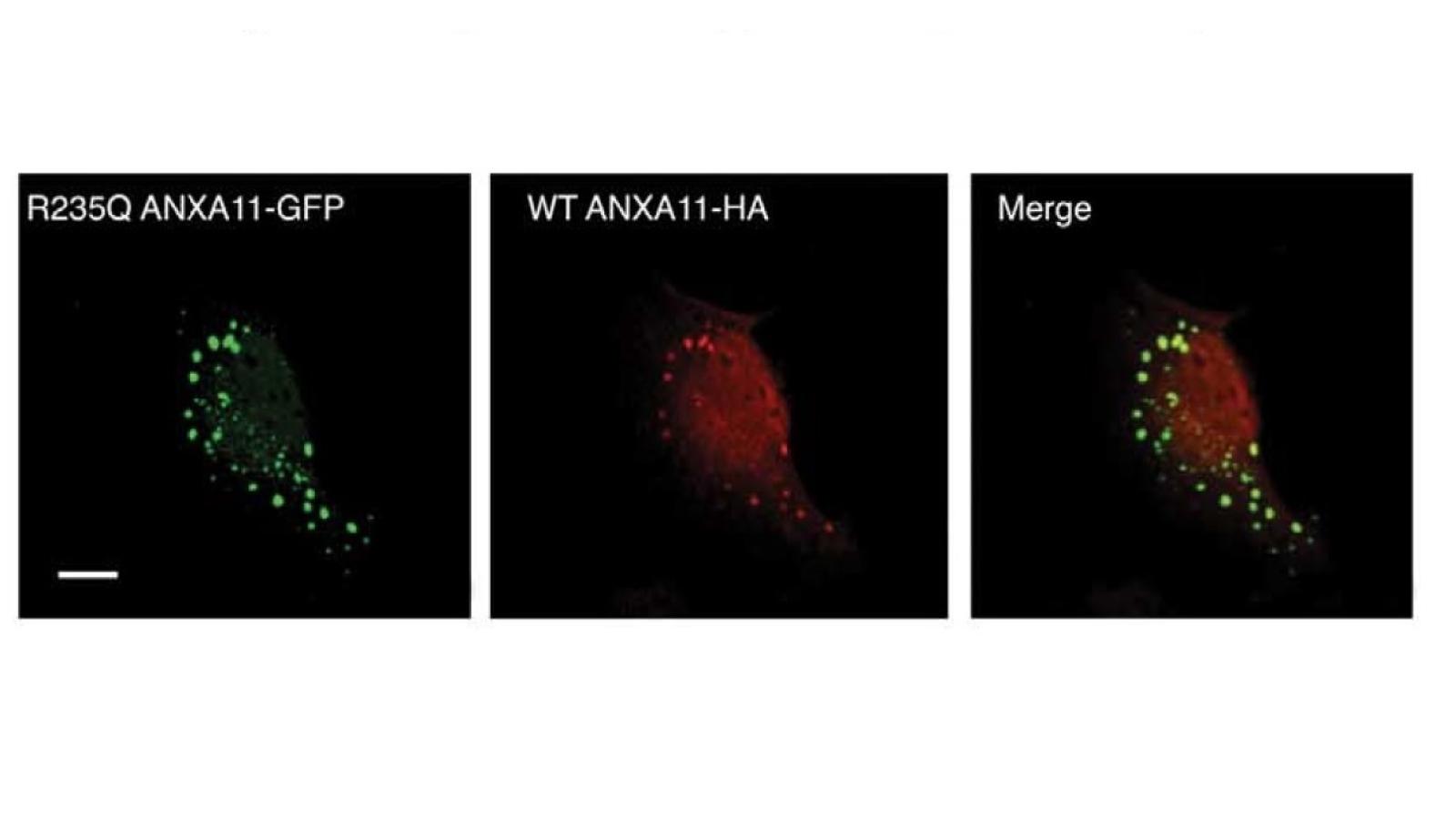
In cell culture studies they subsequently showed that the mutant forms of the protein failed to bind to their usual binding partners and had a tendency to aggregate. Because Annexin A11 plays an important role in trafficking proteins in specialised packages (vesicles) around cells, Professor Shaw's team believe that breakdown of this function leads to a build up of toxic proteins and motor neuron degeneration.
Around a dozen MND risk genes have been discovered previously, many by Professor Shaw's team, but this is the first identification of mutations in Annexin A11 and highlights the important role played by vesicular trafficking.
This finding could bring significant benefits to patients, for example testing for this gene could be offered to patients to help with diagnosis and to at risk family members. It also means that we have a new clues about disease mechanisms which will help in our strategy to develop new treatments.
The team are planning new research supported by the UK DRI to explore the disease mechanisms in stem cells, fruit flies, zebrafish and mice.
The study has been published in Science Translational Medicine.