A novel framework that assesses the progression of Huntington’s disease, has been developed by scientists including Prof Sarah Tabrizi (UK DRI at UCL), as part of an international consortium. The system groups people with the condition according to their underlying biological, clinical, and functional characteristics, and will pave the way for clinical drug trials targeting the earlier stages of disease.
Published in The Lancet Neurology today, the new evidence-based research framework for Huntington’s, called the Huntington’s Disease Integrated Staging System (HD-ISS), includes criteria to biologically define the disease as well as a staging system that encompasses the whole lifespan of disease progression from birth. It is the first time a staging system has been developed for a genetic neurological condition.
The HD-ISS defines groups of people with Huntington’s who have similar prognostic characteristics, aiding research facilitating data comparison across different trials and studies. The researchers hope this will accelerate drug development, and aid in communication between different stakeholders, from families affected by the disease to health policy professionals.
Importantly, this new biological research definition of Huntington’s will allow evaluation of new therapeutic approaches in people during the very early stages of disease, before they start to experience clinical signs. This will likely provide the best chance of substantially slowing disease progression and offer the greatest clinical benefit.
We developed the HD-ISS using state-of-the-art evidence-based methodology and it will revolutionise our ability to evaluate novel disease-modifying therapeutics much earlier in the disease course, when therapies will likely have the best chance of slowing disease progression and providing clinical benefit.Prof Sarah TabriziUK DRI Group Leader at UCL
“Families affected by Huntington’s have long known that signs and symptoms begin decades before the classical motor onset diagnosis that occurs late in the disease course,” said Prof Sarah Tabrizi, UK DRI Group Leader at UCL, and first author of the study. “We developed the HD-ISS using state-of-the-art evidence-based methodology and it will revolutionise our ability to evaluate novel disease-modifying therapeutics much earlier in the disease course, when therapies will likely have the best chance of slowing disease progression and providing clinical benefit. This research required the selflessness and dedication of thousands of research participants, to whom we are immensely grateful.”
Current clinical diagnosis of Huntington’s relies on observation of established clinical signs (primarily involuntary motor signs, but also cognitive impairment and behavioral changes) that emerge late in disease course. This diagnostic approach was developed before the discovery of the huntingtin gene and the consequent genetic test for the CAG expansion – the gene mutation now known to cause the disease - and before the current understanding of disease-related pathobiological changes that develop many decades before these observable clinical signs.
“As a validated staging system, the HD-ISS has the potential to transform how Huntington’s clinical research is conducted, enabling study of the earliest disease phases and planning of preventive clinical trials, as well as facilitating data aggregation and sharing” said Cristina Sampaio, Chief Clinical Officer at CHDI Management/CHDI Foundation.
The disease phase prior to this current diagnostic stage has been variously described as ‘presymptomatic’, ‘premanifest’, or ‘prodromal’, poorly defined terms that make it difficult to compare data and findings across trials and studies.
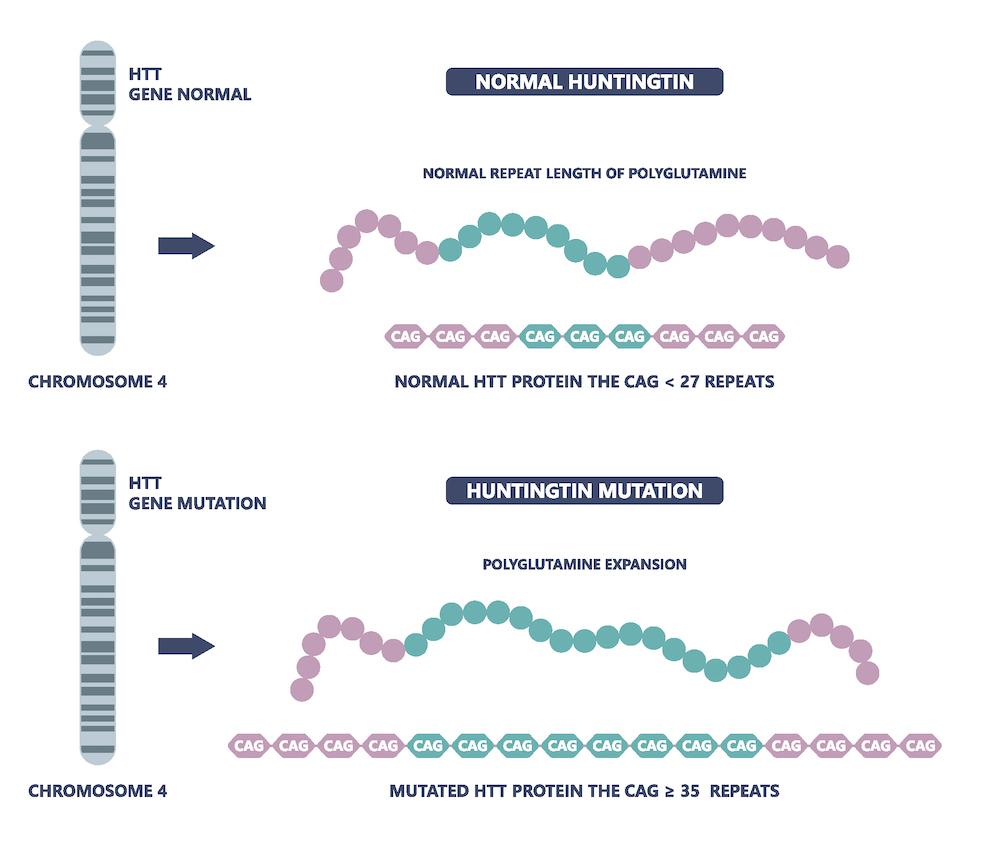
The underlying biological cause of Huntington’s is an increased number of repetitive stretches of the DNA sequence CAG/CTG (CAG repeats)
The HD-ISS includes new staging criteria that cover the entire disease spectrum from birth, beginning at Stage 0 (i.e., individuals with the HD genetic mutation without any detectable pathological change), with subsequent progression marked by measurable indicators of underlying pathophysiology (Stage 1), a detectable clinical phenotype (Stage 2), and then decline in function (Stage 3).
“Ultimately, our goal is to deliver therapeutics at the right time to effectively treat this disease,” said Prof Tabrizi. “Ideally, we’d like to delay or prevent neurodegeneration while function is still intact, giving people with HD many more years without impairment. The HD-ISS is an important tool that will allow us to do this as therapeutics emerge.’’
The HD-ISS was developed within the Huntington’s Disease Regulatory Science Consortium (HD-RSC) under the aegis of the Critical Path Institute (C-Path) by a group of academic researchers, clinician scientists, and representatives from pharmaceutical and biotech companies that have clinical HD programs. The process employed a formal consensus method for the decision-making process that involved systematic investigation and evidence gathering, expert panel debate on the evidence interpretation followed by consensus voting, then finally endorsement by a wide range of stakeholders, including HD family members.
Emily Turner, Executive Director of the HD-RSC at C-Path, said “The HD-ISS truly represents a pivotal, collaborative moment in Huntington's disease clinical research, and the Critical Path Institute is proud to have helped facilitate the development of this much needed staging system.”
Louise Vetter, President & CEO of the Huntington’s Disease Society of America (HDSA), speaking from the 37th Annual HDSA Convention in Atlanta, Georgia where Professor Tabrizi gave the keynote address describing the HD-ISS to HD families, said, “This is a positive and powerful development that brings the lived experience of people with HD to the heart of research and provides a path forward for people earlier in their HD journey to be a part of clinical research. The overwhelming response from HD families has been ‘What took so long?’
Listen to Prof Tabrizi and Cristina Sampaiao discuss the HD-ISS on The Lancet Neurology podcast.
Find out more about Prof Sarah Tabrizi’s research via her UK DRI profile.
Reference: Tabrizi SJ, Schobel S, Gantman EC, Mansbach A, Borowsky B, Konstantinova P, Mestre TA, Panagoulias J, Ross CA, Zauderer M, Mullin AP, Romero K, Sivakumaran S, Turner EC, Long JD, Sampaio C; Huntington's Disease Regulatory Science Consortium (HD-RSC). A biological classification of Huntington's disease: the Integrated Staging System. Lancet Neurol. 2022 Jul;21(7):632-644. doi: 10.1016/S1474-4422(22)00120-X. PMID: 35716693.
Article published: 16 June 2022
Banner image: Dr Rachael Scahill / Track-HD study
