Understanding blood vessel dysfunction in the brain: From rare genetic conditions to dementia
Small blood vessel disease in the brain is a major cause of dementia, but scientists don't fully understand how it works. By studying rare genetic conditions that affect brain blood vessels, researchers can better understand how blood vessel problems lead to brain disease.
One important gene being studied is called TREX1. When this gene is faulty, it can cause two different brain conditions. In one condition, called Aicardi Goutieres Syndrome (AGS), children are born with high levels of inflammatory proteins called interferons in their brain. TREX1 normally helps clean up excess DNA in cells, but when it doesn't work properly, it triggers this harmful inflammation.
The Hunt Lab is investigating how problems with TREX1 damage brain blood vessels in different ways. The team are particularly interested in understanding how high interferon levels affect tiny blood vessels in the brain, as this might explain how brain damage occurs in these conditions. This research could help develop new treatments not only for rare genetic conditions but also for more common forms of dementia caused by blood vessel problems.
Latest news

Prof David Hunt
Prof David Hunt is a Group Leader at the UK DRI at Edinburgh. Find out more about his career and expertise on his profile page.

Research summary
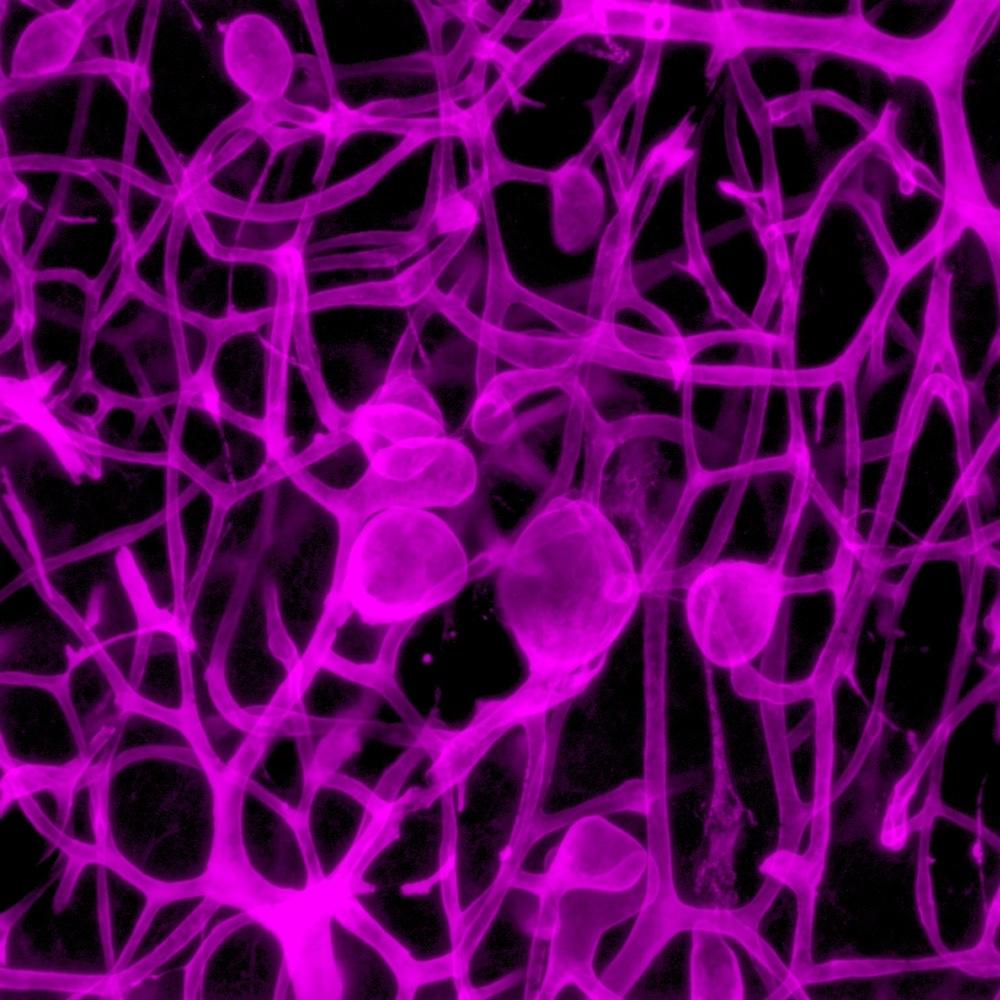
Small blood vessels from mice. Credit: Barney Viengkhou & Markus Hofer
Microvascular mechanisms in cerebral small vessel disease: From monogenic disorders to therapeutic insights
The Hunt Lab investigates the pathogenic mechanisms underlying cerebral small vessel disease (CSVD), a significant but incompletely understood contributor to cognitive decline and dementia. The team focus on TREX1-associated disorders as model systems for understanding how microvascular dysfunction leads to neurological disease.
Loss-of-function mutations in TREX1, the primary human 3'-5' exonuclease, result in distinct genetic brain conditions characterised by prominent cerebral microangiopathy. In Aicardi Goutieres Syndrome (AGS), TREX1 dysfunction leads to accumulation of immunostimulatory nucleic acids and subsequent type I interferon activation. While elevated intracerebral interferon-alpha levels are well-documented in AGS patients, the specific cellular mediators of interferon-induced neurotoxicity, particularly in the context of microvascular dysfunction, remain poorly understood.
Current genetic models based on nuclease dysfunction fail to fully recapitulate AGS neurological phenotypes. However, the GFAP-ifna1 (GIFN) mouse model, which exhibits astrocyte-specific interferon-alpha overexpression, demonstrates key pathological features including microangiopathy, neuronal loss, and calcification. Given previous findings establishing type I interferons as causative factors in small vessel disease, the Hunt Lab hypothesises that cerebral microvasculature may be a critical mediator of interferon-alpha-induced neurotoxicity. The team aims to elucidate the mechanistic links between TREX1 dysfunction, microvascular damage, and neurological deterioration, potentially identifying novel therapeutic targets for both rare genetic CSVDs and more common forms of vascular cognitive impairment.
Key publications

Cerebral interferonopathies such as Aicardi-Goutières’ Syndrome stem from chronic activation of the type I interferon response within the central nervous system, but the mediators of neurotoxicity are poorly defined. Viengkhou et al. identify the cerebral microvasculature as a critical transducer of interferon toxicity within the brain. Deletion of the type I interferon receptor, IFNAR1, on endothelial cells not only rescued cerebral vascular disease and restored blood-brain barrier integrity but also prevented the development of diffuse brain disease, including neurodegeneration. The devastating neurological changes mediated through cytokine-driven microvascular disease are depicted as a leaf, full of tiny, delicate microvasculature networks reminiscent of the human brain, changing from green to autumnal brown. Image by Ben Gartland, Grant Foster, and Holly Philip.
Lab members
- Dr Sarah McGlasson (Postdoctoral Researcher)
- Dr Katy Reid (Postdoctoral Researcher)
- Dr Anna Klingseisen (Postdoctoral Researcher)
- Deborah Forbes (PhD Student)
- Bastien Rioux (PhD Student - Clinical)
Vacancies
Collaborators







Lab funders
Thank you to all those who support the Hunt Lab!