New research led by Prof Karen Duff and Dr Stephanie Fowler (UK DRI at UCL), with Dr Benjamin Ryskeldi-Falcon and Tiana Behr (MRC LMB), provides insight into how toxic tau protein could spread through the brain in Alzheimer’s, via structures called extracellular vesicles. The study, published in the journal Nature Neuroscience, reveals new biological properties for tau, which could lead to new treatment strategies for Alzheimer’s disease.
What was the challenge?
A hallmark of Alzheimer’s is the accumulation of clumps of misfolded tau protein within neurons. These clumps were sometimes found within extracellular vesicles, which are small bubble-like structures that can transfer materials between cells.
Scientists believe that these vesicles might be used by brain cells to throw out toxic tau, in the same way that rubbish is packaged into bin bags and left outside to be cleared away. However, once outside the cell, instead of being removed, the vesicles can be picked up by neighboring cells and spread their toxic contents throughout the brain.
This discovery provides a roadmap for future research into how tau spreads and ways to intervene, bringing us closer to new diagnostic and treatment strategies for Alzheimer’s.
Centre Director
What did the team do and what did they find?
In the study, the team used a combination of proteomic tools and advanced imaging techniques to analyse extracellular vesicles from the brains of people with Alzheimer’s, identifying the contents of vesicles and creating detailed images of the structure of the tau within them and how it was organised. They then showed that the vesicles could spread tau to new neurons.
They discovered that:
- The vesicles contained specific fragments of tau which are thought to be more toxic and harder for the cell to handle.
- The vesicles come from a part of the cell called the endo-lysosomal system, responsible for the recycling and breakdown of unwanted molecules - suggesting the tau clumps may end up in extracellular vesicles when they are unable to be broken down via the cells usual degradation systems.
- The tau in vesicles had a similar but not identical structure to tau in the rest of the brain of an Alzheimer’s patient, indicating new ways to understand how tau structures form.
- Tau seemed to be tethered, or attached, to the vesicle walls, rather than free floating within the structures. This new finding has implications for how the tau fragments form or get into the vesicles.
- The tau within extracellular vesicles could infect neighbouring cells indicating that it could be a means by which toxic tau spreads through the brain, leading to progressive worsening of the disease.
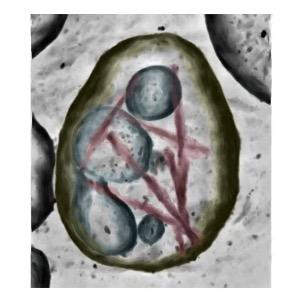
Cryo-ET of a brain extracellular vesicle from an individual with AD containing tau filaments (magenta) tethered to the limiting membrane (yellow). Credit: MRC LMB and Duff Lab, UK DRI at UCL
What is the impact?
These findings could help scientists better understand how toxic fragments of tau form, and how they spread through the brain. This could lead to therapies that block tau spread, potentially slowing or halting disease progression. It may also provide new ways to detect Alzheimer’s early by identifying tau-carrying vesicles. Targeting the mechanism by which tau attaches to the vesicles could be a strategy to modulate the formation of toxic tau filaments and the spread of tau via extracellular vesicles.
Dr Stephanie Fowler, now a Fellow at the Oxford-GSK Institute of Molecular and Computational Medicine (IMCM), explained:
“We have shown that these forms of tau are attached to the walls of extracellular vesicles, that are rich in endo-lysosomal proteins. Our work opens up new opportunities to study how these clumps of tau protein form and are released from cells, and to develop strategies to target them.”
Prof Karen Duff added:
“Our study reveals how toxic tau fragments are selectively packaged into tiny cellular vesicles, offering new insights into their role in Alzheimer’s disease progression. This discovery provides a roadmap for future research into how tau spreads and ways to intervene, bringing us closer to new diagnostic and treatment strategies for Alzheimer’s."
Reference: Fowler, S.L., Behr, T.S., Turkes, E. et al. Tau filaments are tethered within brain extracellular vesicles in Alzheimer’s disease. Nat Neurosci (2024). https://doi.org/10.1038/s41593-024-01801-5
Molecular mechanisms
Read about how UK DRI researchers are investigating the intricate processes maintaining health and driving neurodegeneration