Adeno-associated virus (AAV) is one of the most actively investigated gene therapy vehicles. Now, under the leadership of Prof Chris Shaw, Associate Director of UK DRI at Kings, the institute will be at the forefront of realising this potential. This programme has been awarded the first in a series of Directors’ Strategic Initiatives from the UK DRI.
In recent years there has been a resurgence in gene therapy efforts that is partly fuelled by the identification and understanding of new gene delivery vectors. AAV is a non-enveloped virus that can be engineered to deliver DNA to target cells, and has attracted a significant amount of attention in the field, especially in clinical-stage experimental therapeutic strategies. The ability to generate recombinant AAV particles lacking any viral genes and containing DNA sequences of interest for various clinical applications is a promising strategy for gene therapies.
The remarkable results of recent clinical trials using AAV vectors to enhance survival motor neuron gene expression in Type 1 spinal muscular atrophy (SMA) have given renewed impetus to deliver more effective therapies for neurodegenerative disorders. For example, AAV-9-SMN therapy resulted in the preservation of muscle function and ventilation-free survival which are big steps towards an eventual “cure”.
This new project at the UK DRI aims to establish a platform across the institute for the design and pre-clinical validation and clinical trials of AAV vectors capable of delivering long lasting, cost effective and safe therapies for neurodegenerative diseases.
Dr Adrian Ivinson, UK DRI Director and COO says:
“The gene therapy field is undergoing a renaissance. At UK DRI we believe in taking big risks that could reap big rewards. Exploring AAV is a great example of this – it will challenge and push us. We hope by being bold it will be transformative.”Building an AAV core facility
Prof Chris Shaw will lead the set-up of a core facility to support the generation of gene therapy vectors for UK DRI group leaders. The core team will advise on the design of gene constructs (to optimise transduction and dosage) and the choice of cellular and animal models and assays to demonstrate target engagement. The team will clone a range of plasmid constructs to be tested in the group leader’s laboratory in preclinical studies. The best construct identified from their cellular assays will be packaged into research-grade AAV vectors suitable for in vivo experimentation, again performed in the appropriate group leader’s laboratory.
When the final construct and AAV vector has been chosen the core team will help them source from an external CRO GLP-grade vector for toxicology studies. In parallel, the group will explore systems for cell-type specificity (e.g. microglial expression), an inducible gene expression (e.g. antibiotic induced expression) and transcript knock down (modified short hairpin RNA,CRISPR/Cas) that can be miniaturized and packaged into an AAV vector.
Prof Chris Shaw, Associate Director, UK DRI at Kings says:
“We’re looking forward to building a key resource for the institute – pushing us further and enabling innovative research in gene therapy. In addition to vector production, our unit will pioneer new technologies for use in vectors.”Frontotemporal dementia: Putting AAV vectors into practice
Alongside building this platform, Chris will use AAV vectors to explore their potential in frontotemporal dementia (FTD) and ALS therapy.
The team - Chris Shaw (Kings) in collaboration with Jonathan Rohrer (UCL), Farzin Farzaneh (KCL) and Dr Younbok Lee (KCL) – will be trialing the system in cell culture, animal models, through to eventual first-in-human, benefiting from access to a unique clinical resource of the Genetic FTD Initiative (GENFI).
The study will initially focus on the development of an AAV vector able to restore levels of the protein progranulin encoded by the GRN gene, which is mutated in about 20% of patients affected by FTD.
Restoration of progranulin levels after viral delivery can be assessed in blood and cerebrospinal fluid. Specialised MRI imaging approaches able to detect brain volumetric loss will be used to test whether AAV9-GRN has an impact on disease progression. If successful, this project may pave the way for novel AAV-based effective therapeutic strategies that could be applied to other neurodegenerative disorders.
Prof Chris Shaw, Associate Director, UK DRI at Kings says:
“The ability to stop, or slow, FTD progression in patients by administration of a single shot vector would represent substantial progress to the FTD field – and give added impetus to this approach in the field of neurodegenerative disease.”
The facility is currently being set up and purchasing equipment and is due to be ‘open for business’ from September 2019.
The first of our Directors’ Strategic initiativesLaunched in 2019, this initiative invites our Associate Directors across the institute to propose a project of strategic relevance to the UK DRI nationally. These projects will capture new ground, bring a new capacity or direction and promote cross-centre collaborative efforts. By their very nature, these initiatives are novel, ambitious, involve or support multiple centres and have a long-term vision of development. To fit with the institute’s core mission, the initiative must have a translational goal. Projects of this scale and ambition need appropriate funding: each will receive up to £2m over four years, with rigorous selection and assessment processes.
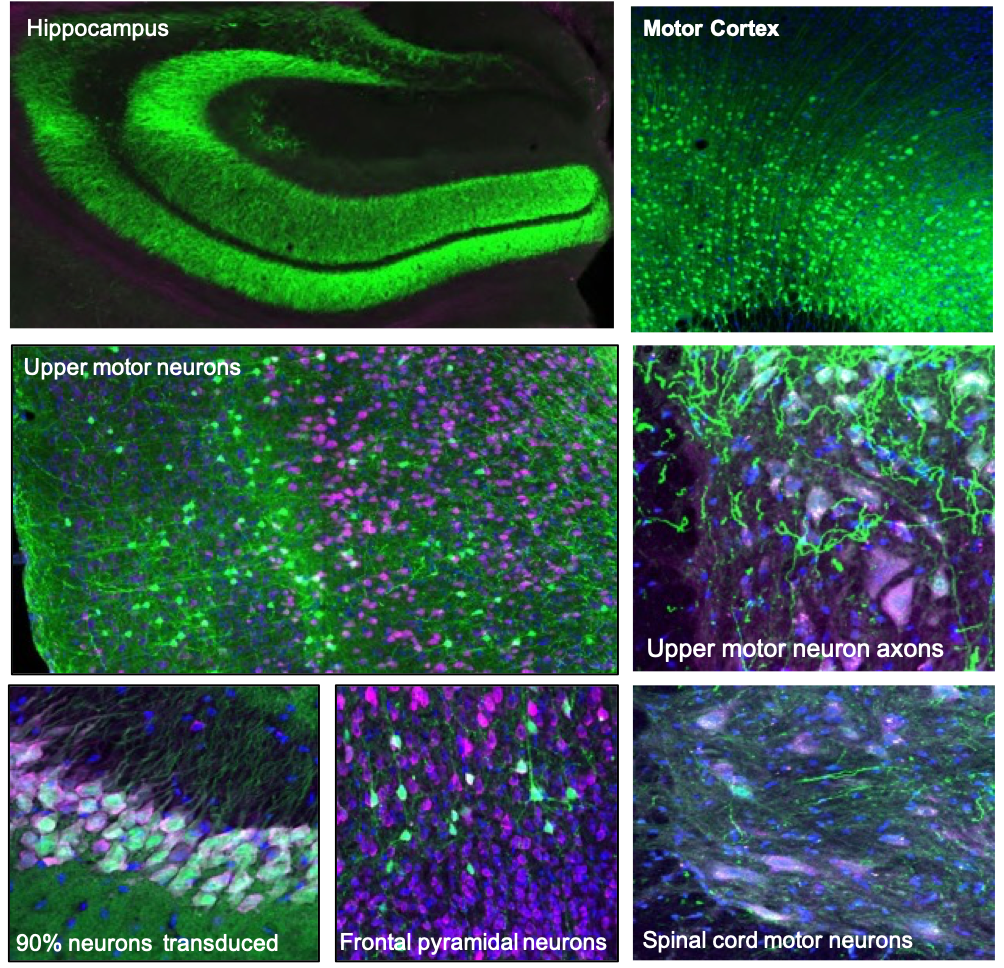
GFP-green neurons transduced by AAV viral vector. Pink neuron staining.
