Detailed brain cell analysis has helped researchers uncover new mechanisms thought to underlie Parkinson’s disease. A large team came together across the UK and US, including UK DRI centres in Cambridge and Edinburgh.
The study, published in Nature Communications, adds to our growing understanding of the causes of Parkinson’s and other neurodegenerative diseases, and could influence drug design in the future.
For years, scientists have known that Parkinson’s disease is associated with a build-up of alpha-synuclein protein inside brain cells. But how these protein clumps cause neurons to die was a mystery.
Using a combination of detailed cellular and molecular approaches to compare healthy and clumped forms of alpha-synuclein, a team of scientists at the Francis Crick Institute, UCL, the UK Dementia Research Institute at the University of Cambridge and the University of Edinburgh, New York University and other collaborators have discovered how the protein clumps are toxic to neurons.
They found that in neurons, clumps of alpha-synuclein moved to and damaged key proteins on the surface of mitochondria - the energy powerhouses of cells - making them less efficient at producing energy. It also triggered a channel on the surface of mitochondria to open, causing them to swell and burst, leaking out chemicals that tell the cell to die.
These findings were replicated in human brain cells, generated from skin cells of patients with a mutation in the alpha-synuclein gene, which causes early-onset Parkinson’s disease. By turning patient skin cells into stem cells, they could chemically guide them into become brain cells that could be studied in the lab. This cutting-edge technique provides a valuable insight into the earliest stages of neurodegeneration - something that brain scans and post-mortem analysis cannot capture.
Dr Sonia Gandhi, Group Leader at the Crick, and joint senior author of the study said: “Our findings give us huge insight into why protein clumping is so damaging in Parkinson’s, and highlight the need to develop therapies against the toxic form of alpha-synuclein, not the healthy non-clumped form.”
Professor Andrey Abramov, joint senior author of the paper said: “This study was a complex collaboration at the interface of chemistry, biophysics and biology, bringing scientists from different disciplines together to investigate a longstanding problem in Parkinson’s research.”
people are living with Parkinson's in the UK
There are many different species of alpha-synuclein, and it's therefore a challenge to know which one is a good biomarker or therapeutic target. Using highly resolved methods to study these at the nanoscale, we have helped demonstrate which form of alpha-synuclein is damaging to cells and therefore this may guide therapy in the future. To achieve this, we brought together a team from multiple disciplines, across different universities and a number of UK DRI centres. Harnessing the breakthroughs in single-molecule methods, single cell imaging and stem cell biology, we have been able to take a multifaceted approach to resolve a significant problem in neurodegeneration.UK DRI FellowMathew Horrocks
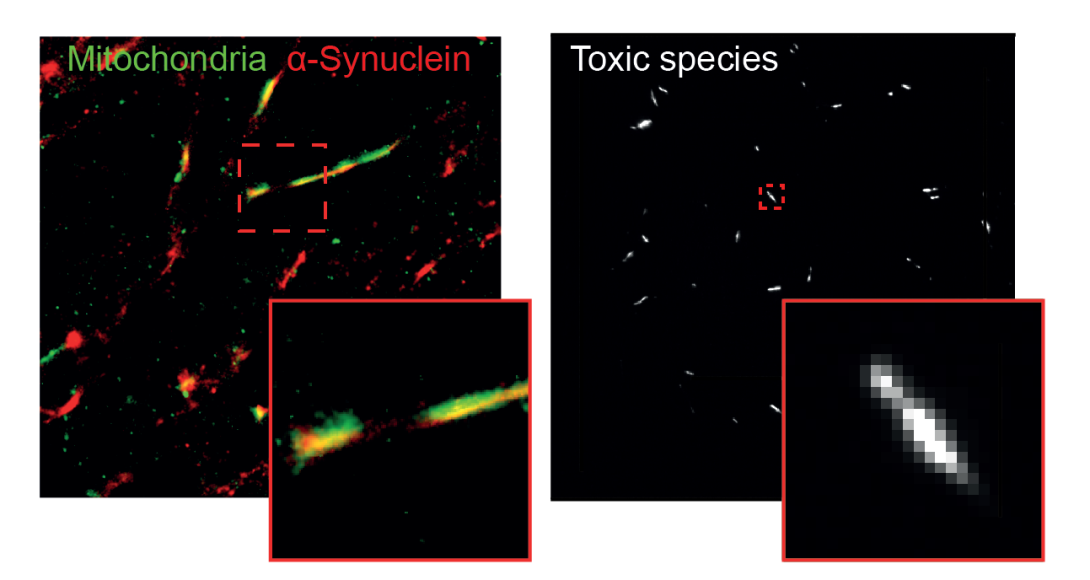
Left: super-resolution image of α-synuclein in mitochondria of neuron. Right: single-molecule image of individual α-synuclein aggregates
