Research led by Dr Soyon Hong (UK DRI at UCL) has uncovered a new molecular mechanism underlying synapse loss in Alzheimer’s disease. The study, published in Nature Neuroscience, could provide new targets for future drug discovery.
During Alzheimer’s disease the connections between neurons, ‘synapses’ are lost, and this correlates strongly with cognitive decline. Loss of synapses can result from the dysfunctional activity of microglia, the resident brain immune cells, which are triggered to engulf synapses in the early stages of Alzheimer’s, in a process called phagocytosis. The signals responsible for driving this process are not well understood.
In the new study, Dr Sebastiaan De Schepper, a postdoctoral fellow in the Hong lab and a Sir Henry Wellcome fellow, identified a role for a protein known as SPP1, or osteopontin, in mediating the loss of synapses in mouse models of Alzheimer’s.
SPP1, also known as osteopontin, mediates synapse loss in Alzheimer's
We know that osteopontin is elevated in the spinal fluid of Alzheimer’s patients, but its function was previously unknown. Our study demonstrates a clear role for the protein in driving the dysfunctional activity of microglia which leads to synapse loss.Dr Soyon HongGroup Leader at the UK DRI at UCL
In mouse models where osteopontin was absent, microglia were prevented from engulfing synapses, indicating that the protein is required for microglial phagocytosis to take place.
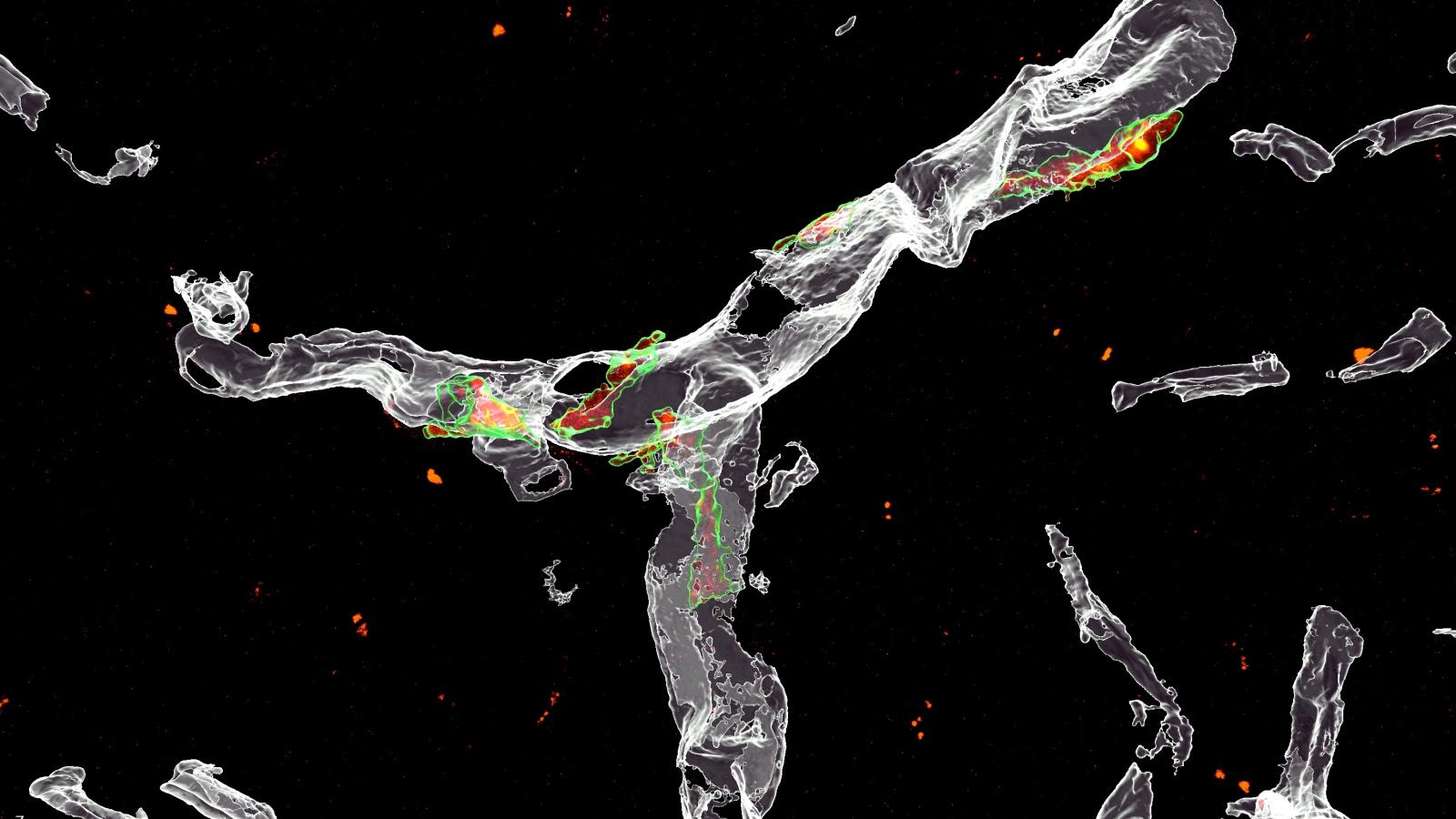
In addition, Dr De Schepper and the team also showed for the first time that microglia interact with cells in the perivascular space, a fluid-filled structure that surrounds small blood vessels in the brain. They discovered that osteopontin is released by cells in the perivascular space, and acts as a signal to microglia to engulf synapses. These cells, known as perivascular macrophages, may act as a first line of defence against harmful agents and pathogens, and now the authors suggest may become activated as amyloid-beta accumulate in the blood vessels.
Dr Hong continued:
“It is becoming increasingly clear that the vascular space is central to Alzheimer’s disease pathology. In fact, blood vessels may represent an early and vulnerable site for amyloid-beta protein deposits to form. Our study suggests that this may be a trigger for an increase in osteopontin levels, which in turn leads to loss of neighbouring synapses and activation of microglia and other brain macrophages.”
The team will now test ways to therapeutically target osteopontin in the early stages of Alzheimer’s, aiming to prevent synapse loss and inflammation in the brain.
To find out more about Dr Soyon Hong’s work, visit her UK DRI profile. To stay up to date on the latest research news and institute updates, sign up to receive our monthly newsletter, ‘Inside Eye on UK DRI’.
This paper was also highlighted in a Nature News & Views article which you can read here.
Reference: De Schepper, S., Ge, J.Z., Crowley, G. et al. Perivascular cells induce microglial phagocytic states and synaptic engulfment via SPP1 in mouse models of Alzheimer’s disease. Nat Neurosci (2023). https://doi.org/10.1038/s41593...
Article published: 7 February 2023
Banner image credit: Dr De Schepper