Researchers led by Prof Adrian Isaacs (UK DRI Group Leader at UCL) have developed a way to monitor the success of therapies targeting the most common genetic cause of frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS). The test has been recently utilised in an early-stage clinical trial to evaluate a new treatment for the diseases.
Biomarkers, short for ‘biological markers’, are a measure – or a flag – of a biological state, helping us identify and monitor healthy biological processes or, crucially, harmful changes occurring in disease.
Prof Henrik Zetterberg, UK DRI Group Leader at UCL, and a collaborator on the study, said:
“In neurodegenerative diseases, biomarkers are particularly important. The diseases are complicated and clinical symptoms appear when neuronal networks break down, which takes time. Direct markers of the molecular processes that cause the disease may change rapidly in response to disease-modifying therapies and may predict rather than reflect clinical onset and disease progression.”
In this recent study, published in the Journal of Neurology, Neurosurgery and Psychiatry, the team set out to develop a biomarker test for the presence of an aberrant protein generated by a faulty copy of the C9orf72 (C9) gene.
In people with FTD and ALS the C9 gene can be mutated or faulty. A large section of DNA is repeated, leading to the production of a dysfunctional protein. This mutation is responsible for approximately 25% of familial FTD cases and 40% of familial ALS cases, causing debilitating symptoms such as progressive cognitive decline in FTD and deterioration of muscle control in ALS.
In efforts to treat FTD and ALS, researchers have developed methods to interfere with the faulty C9 gene and stop the production of these abnormal proteins. One therapeutic approach involves delivering a single strand of DNA, known as an ‘antisense oligonucleotide’, into targeted cells. This oligonucleotide specifically binds messenger RNA (mRNA), which is crucial for translating DNA code into new proteins, blocking this process and reducing the amount of abnormal protein produced.
Studies using this technique are now underway (including at UCL) and represent the first time such patients have been able to enter into a trial that could potentially treat such a devastating illness. However, a significant challenge is knowing whether the antisense oligonucleotide therapy has succeeded in disrupting the generation of the faulty protein.
of familial FTD cases & 40% of familial ALS cases are caused by the C9 gene mutation
To find a test that correctly detects the presence of a protein only in people with FTD and ALS is really quite rare, so we were very pleased with this result.Prof Adrian IsaacsUK DRI Group Leader at UCL
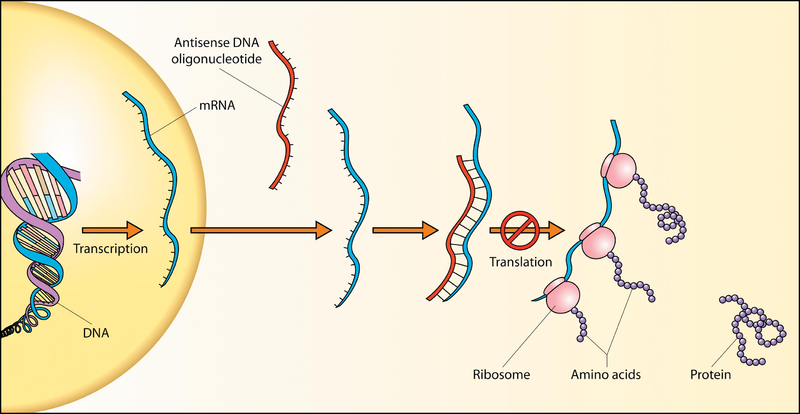
DNA oligonucleotides block translation and prevent abnormal proteins being produced
Prof Isaacs and his team aimed to develop a test sensitive enough to detect and monitor the low levels of abnormal C9 protein present in spinal fluid, so that treatment to reduce them could be evaluated accurately. Collaborating with Prof Zetterberg, an expert in neurodegenerative biomarkers and lead on the UK DRI Biomarker factory, the team used ultra-sensitive technology – known as Simoa (single molecule array) – to detect the proteins at the extremely low levels needed, in the spinal fluid of people with ALS and FTD.
The team also collaborated with Prof Jonathan Rohrer (Chief investigator of the GENFI study and UK DRI Clinical Co-investigator at UCL) to assess the test in a real-world clinical setting. They found that it was able to correctly detect the protein in 100% of people who had the gene mutation, compared to healthy individuals, meaning the test was extremely specific and sensitive.
Prof Isaacs said: “To find a test that correctly detects the presence of a protein only in people with FTD and ALS is really quite rare, so we were very pleased with this result.”
Excitingly, the biomarker test has already been harnessed for a clinical trial to assess the effectiveness of a new therapy for FTD and ALS. Results from the proof-of-concept study, led by pharmaceutical company Wave Life Sciences, show that WVE-004 stereopure antisense oligonucleotide successfully reduces the amount of abnormal protein present in the spinal fluid of people with ALS and FTD. The researchers plan to continue testing the therapy in a larger cohort of people to assess if it has a beneficial effect on symptoms and disease progression.
Michael Panzara, MD, MPH, Chief Medical Officer and Head of Therapeutics Discovery and Development at Wave Life Sciences said:
“Our collaboration with the UCL to develop the poly(GP) test allowed us to use an innovative study design to confirm the biological effect of single doses of WVE-004 in a small number of patients, and then adapt the clinical trial to further optimize dose level and frequency while the study is ongoing. The impact for patients is the potential to develop a new treatment for these devastating diseases much earlier than might otherwise be possible.”
Prof Isaacs added: “It’s super exciting to think that this therapy may go on to become a successful treatment for these diseases, and the trial wouldn’t have been possible without the use of our test.”
Read more about the work of Prof Adrian Isaacs on his UK DRI profile.
Reference: Wilson KM, Katona E, Glaria I, et al Development of a sensitive trial-ready poly(GP) CSF biomarker assay for C9orf72-associated frontotemporal dementia and amyotrophic lateral sclerosis Journal of Neurology, Neurosurgery & Psychiatry Published Online First: 04 April 2022. doi: 10.1136/jnnp-2021-328710
Article published: 13 May 2022
Banner image: Shutterstock/ CI Photos
Antisense oligonucleotide diagram source: RNAi Therapeutics: How Likely, How Soon? Robinson R PLoS Biology Vol. 2, No. 1, e28 doi:10.1371/journal.pbio.0020028