As we unpick the underlying causes of neurodegeneration, the emerging picture is getting more complex with the involvement of several players and mechanisms. This offers great opportunity for treatment at different stages of disease, however, there is still a significant challenge in selecting viable components and pathways for drug targeting. We spoke to Dr Emmanouil Metzakopian, Group Leader from UK DRI at Cambridge, who is harnessing and combining the latest genetic technologies to identify these promising candidates, with the ultimate aim of collaborating with industry and developing much-needed treatments for dementia.
Rebalancing and protecting cells
In neurodegenerative diseases such as Parkinson’s, toxic proteins build up in the brain which provoke stress responses in our cells. Chronic activation of these stress pathways causes an imbalance in the cell, leaving it unable to carry out basic functions such as clearance of these harmful proteins. Dr Metzakopian is aiming to address this issue by interrogating the genome and finding biological pathways that re-balance this cellular disease state early in the process, protecting neurons from dysfunction and subsequent degeneration.
“The dysfunction and loss of synaptic connections in the brain appears to be an early event in the neurodegenerative process. We’re keen to understand the disease mechanisms behind this in Alzheimer’s and Parkinson’s, with a particular focus on the activation of stress pathways within neurons.”
Harnessing the latest technology
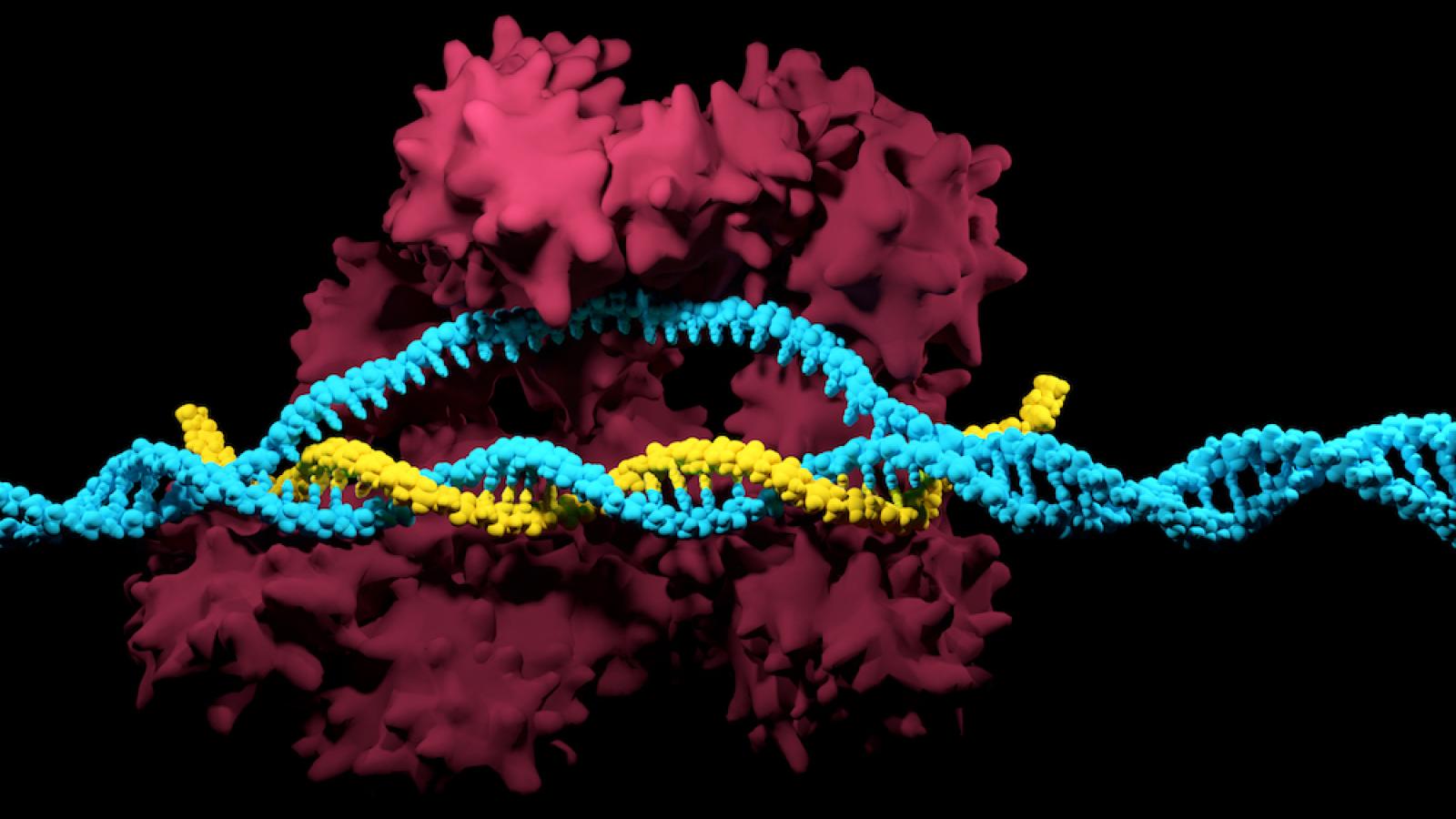
Dr Metzakopian is taking advantage of a revolutionary technology developed over the past decade – called CRISPR-Cas9. CRISPR has been transformational in providing scientists world-wide with a relatively simple and inexpensive method to edit genomes for a seemingly infinite number of applications. With expertise in stem cell technology, Dr Metzakopian saw an opportunity to use CRISPR-Cas9 to mutate single genes within individual cells, to be able to assess functional consequences and identify potential leads for drug discovery.
CRISPR-Cas9 works like a pair of molecular scissors, cutting DNA strands at very specific sequences. Once cut, new DNA can be inserted to change the sequence, or a gene can be knocked out altogether by taking advantage of inevitable errors in the DNA repair process. The specificity of CRISPR-Cas9 is achieved by design of a unique section of guide RNA, which directs the Cas9 enzyme to the correct part of the genome to cut.
While at the Wellcome Sanger Institute before moving to the UK DRI, Dr Metzakopian and colleagues created an arrayed library of these guide RNAs for the whole human and mouse genome. This extraordinary and comprehensive gene editing toolbox, which covers 17,166 and 20,430 genes in human and mouse respectively, allows researchers to select the gene of interest they wish to mutate and simply pick out the corresponding guide RNA to begin mutating their cellular model. After teaming up with Merck for distribution, this resource is widely available to labs across the globe.
Emmanuelle Charpentier and Jennifer A. Doudna were awarded the Nobel Prize in Chemistry in 2021 for their role in pioneering the revolutionary gene-editing technology
We’re now reaching a really exciting stage where we have promising, validated gene candidates that we want to take forward for drug discovery.Dr Emmanouil MetzakopianUK DRI at Cambridge
Screening the genome
At his lab in the UK DRI at Cambridge, Dr Metzakopian now uses these methods to screen the genome and prioritise genes of interest. The first step in this process is to take a pooled library of guide RNAs from the ‘druggable genome’ and disable single genes in human induced pluripotent stem cell (IPSC) derived neurons. The resulting mutant neurons are then exposed to a toxin, for example one that provokes oxidative stress, to see if the gene’s removal protects the cell and allows it to survive. DNA from the resistant cells is sequenced to identify the gene of interest, and then further tests carried out to assess whether the target gene should be prioritised.
“From a long list of genes involved in neuronal survival to the stressor toxin, you can select out the ones found in pathways relevant to oxidative stress. For example, when you see genes linked to mitochondria, DNA damage etc, you know these are good targets to take forward. Next, you validate these genes again by subjecting the cells to further tests with multiple steps and readouts. We are not only interested in genes that can rescue cells from death, but also those that maintain neuronal connectivity, activity and functionality. If you can identify good validation tests, it’s a fantastic platform method to assess a whole range of cellular characteristics and the genes influencing them.”
Dr Metzakopian has been able to perform multiple screens with numerous stressors relevant to neurodegenerative disease. With a manageable list of interest genes acquired, the team can probe interactions, pathways and downstream mechanisms, before further validation with animal models. Again, with CRISPR-Cas9, new mouse models can be created by knocking out genes in specific cells, for example dopaminergic neurons, before assessing survival of this cell population when exposed to a stress stimulus such as the toxic protein alpha synuclein.
“CRISPR-Cas9 technology has been a really huge breakthrough and that's why it deservedly received the Nobel prize in 2020. With a specific and simple method to generate mutant cells, it allows us to cast a wide net over the genome, identifying a good number of gene targets that can be validated rapidly in cell and animal models. We’re now reaching a really exciting stage where we have promising, validated gene candidates that we want to take forward for drug discovery.”
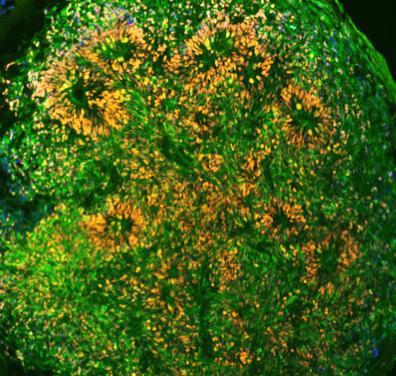
Midbrain organoids (30-days old) produced by PhD student Rizwan Ansari in the Metzakopian lab. The yellow staining shows co-localisation of Foxa2 (red - a floor plate
Where can this take us?
Dr Metzakopian is keen to innovate further with ambitions to improve on methods and models. The lab has established brain organoids, spherical 3D cultures with the potential to encompass multiple cells types in conditions that better mimic the brain microenvironment.
“With further resources and investment, the sky’s the limit with these methods. I think this is a perfect opportunity for academia and industry to work together on accelerating and improving validation. We are already looking at increasing the speed of target gene identification, expansion into multiple models and cell types and higher throughput at the animal validation stage. We could even begin to look at setting up small molecule design and validation for drug discovery.”
For more information on the work of Dr Emmanouil Metzakopian or to explore partnering opportunities with any of the UK DRI Group Leaders, please contact UK DRI Director of Innovation and Business, Dr Kay Penicud.
Article published: 23 April 2021
Banner image: Meletios Verras/Shutterstock.com