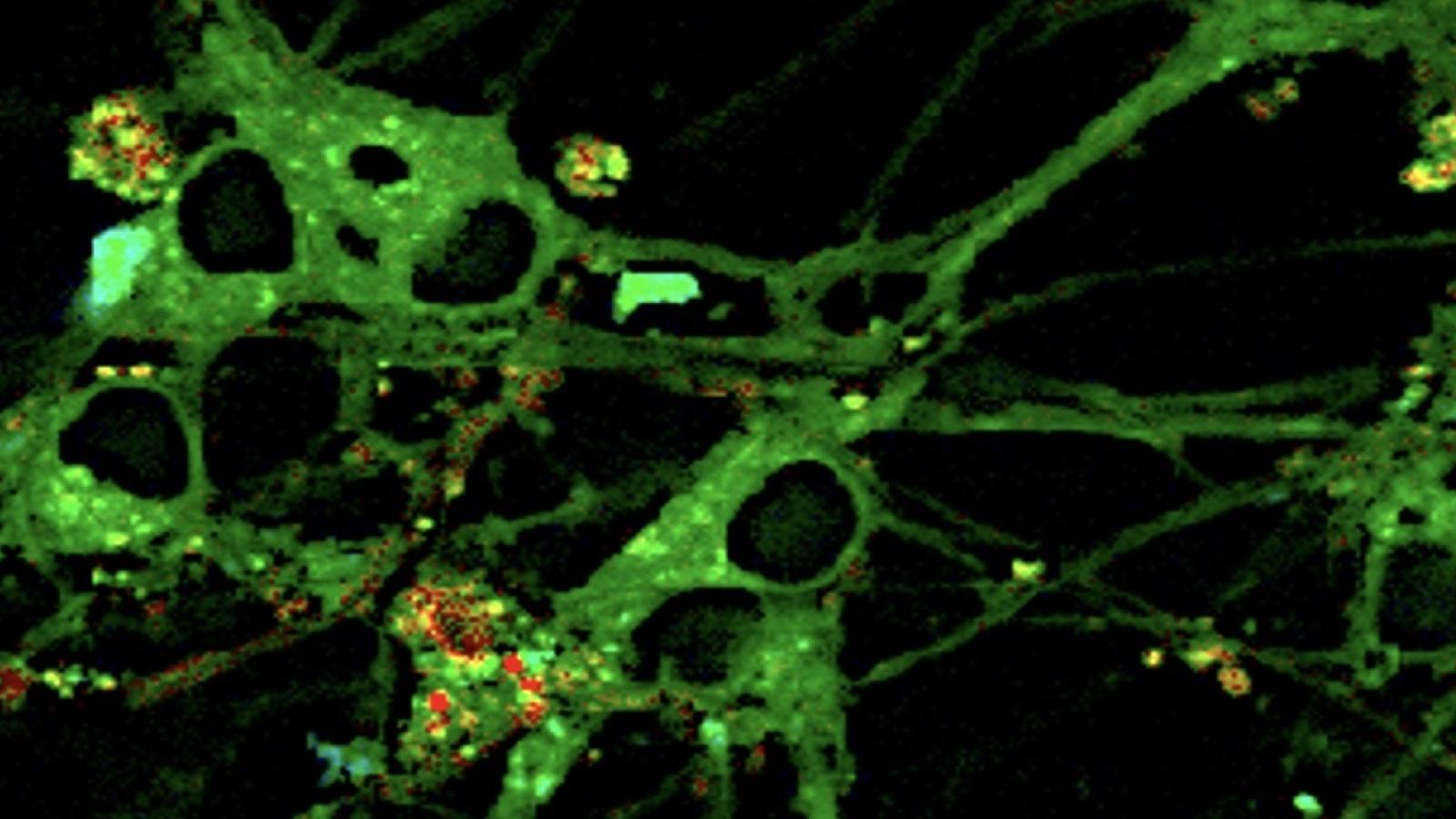
A study led by Dr Edward Avezov (UK DRI at Cambridge) has uncovered a new mechanism that might help prevent the build-up of tangles of proteins commonly seen in dementia.
Protein folding is a critical process in our body’s cells, and to ensure this is carried out correctly, a form of quality control takes place to destroy proteins that are misfolded. In neurodegenerative diseases such as Alzheimer’s and Parkinson’s, this system becomes impaired, causing a build-up of misfolded proteins that form clumps or ‘aggregates’, leading to irreversible damage to cells in the brain.
In the study, published today in Nature Communications, the team from the UK DRI at Cambridge discovered a mechanism that appeared to reverse the build-up of misfolded proteins, not by eliminating them completely, but rather by ‘refolding’ them. Dr Avezov and colleagues found that placing cells under mild stress initiated this mechanism of refolding.
If we can find a way of awakening this mechanism without stressing the cells – which could cause more damage than good – then we might be able to find a way of treating some dementias.Dr Edward AvezovGroup Leader
“Just like when we get stressed by a heavy workload, so, too, cells can get ‘stressed’ if they’re called upon to produce a large amount of proteins,” explained Dr Avezov.
“There are many reasons why this might be, for example when they are producing antibodies in response to an infection. We focused on stressing a component of cells known as the endoplasmic reticulum, which is responsible for producing around a third of our proteins – and assumed that this stress might cause misfolding.”
The endoplasmic reticulum (ER) is a membrane structure found in mammalian cells. It carries out a number of important functions, including the synthesis, folding, modification and transport of proteins needed on the surface or outside the cell. Dr Avezov and colleagues hypothesised that stressing the ER might lead to protein misfolding and aggregation by diminishing its ability to function correctly, leading to increased aggregation. They were surprised to discover the opposite was true.
“We were astonished to find that stressing the cell actually eliminated the aggregates – not by degrading them or clearing them out, but by unravelling the aggregates, potentially allowing them to refold correctly,” said Dr Avezov.
“If we can find a way of awakening this mechanism without stressing the cells – which could cause more damage than good – then we might be able to find a way of treating some dementias.”
of proteins in mammalian cells are made in the endoplasmic reticulum
The main component of this mechanism appears to be one of a class of proteins known as heat shock proteins (HSPs), more of which are made when cells are exposed to temperatures above their normal growth temperature, and in response to stress.
Dr Avezov speculates that this might help explain one of the more unusual observations within the field of dementia research. “There have been some studies recently of people in Scandinavian countries who regularly use saunas, suggesting that they may be at lower risk of developing dementia. One possible explanation for this is that this mild stress triggers a higher activity of HSPs, helping correct tangled proteins.”
One of the factors that has previous hindered this field of research has been the inability to visualise these processes in live cells. Working with teams from Pennsylvania State University and the University of Algarve, the team has developed a technique that allows them to detect protein misfolding in live cells. It relies on measuring light patterns of a glowing chemical over a scale of nanoseconds - one billionth of a second.
“It’s fascinating how measuring our probe’s fluorescence lifetime on the nanoseconds scale under a laser-powered microscope makes the otherwise invisible aggregates inside the cell obvious,” said Professor Eduardo Melo, one of the leading authors, from the University of Algarve, Portugal.
To find out more about the work of Dr Edward Avezov, take a look at his profile page.
Sign up to the UK DRI newsletter to keep up to date on the latest news, research and public events from the Institute.
Reference: Melo, EP, et al. Stress-induced protein disaggregation in the Endoplasmic Reticulum catalysed by BiP. Nature Comms; 6 May 2022; DOI: 10.1038/s41467-022-30238-2
Article published: 6 May 2022
Image and video credit: Edward Avezov
Video shows a three-dimensional reconstruction of the endoplasmic reticulum of a live cell showing protein aggregates in yellow, and normal proteins in green.