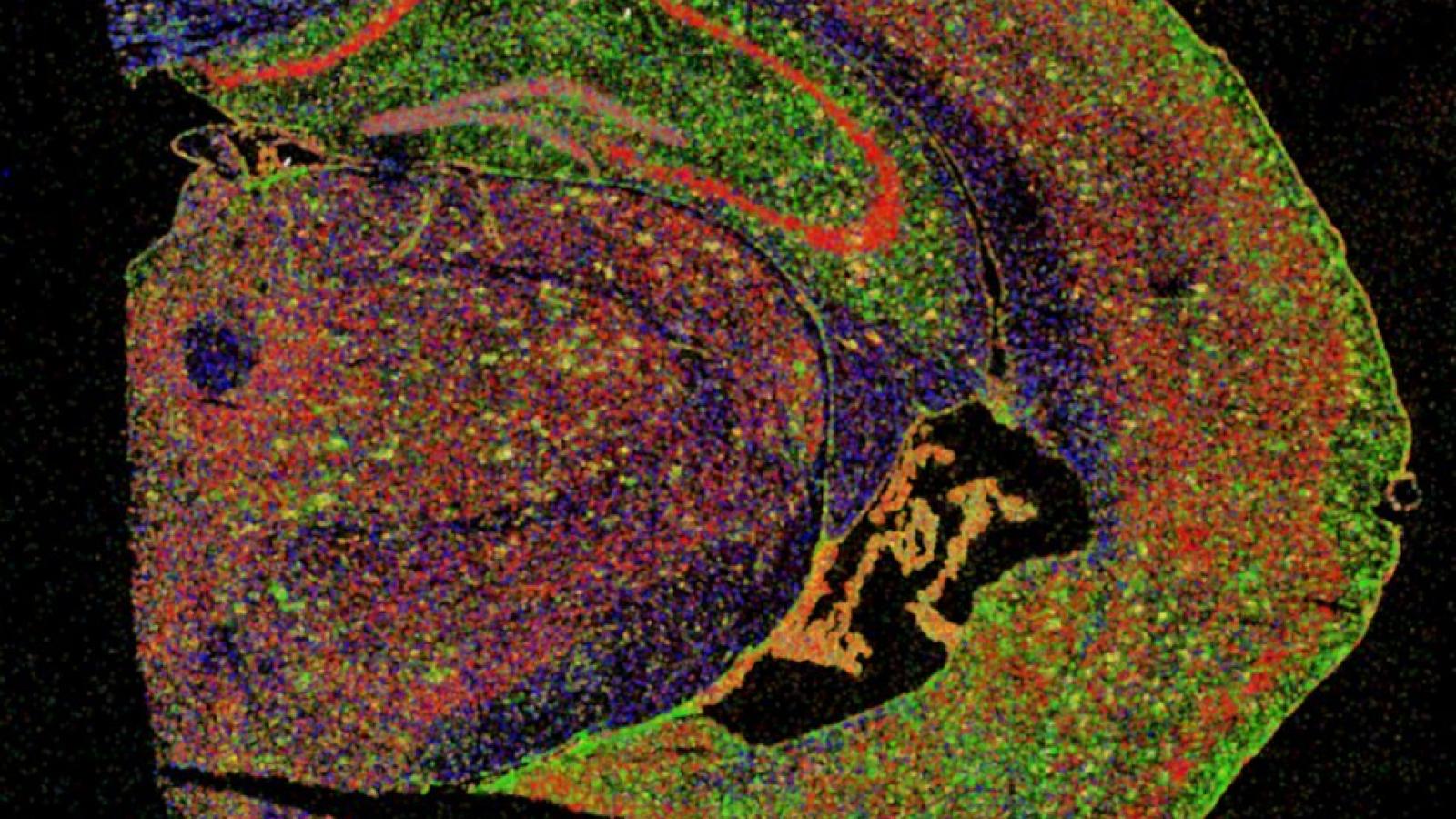
Researchers led by Prof Bart De Strooper (UK DRI Director) and Dr Mark Fiers at the VIB-KU Leuven Center for Brain & Disease Research in Leuven, Belgium, have used pioneering technologies to investigate what happens in brain cells in the direct vicinity of protein plaques in Alzheimer’s disease. Using a mouse model of the condition and human brain tissue, the team’s findings suggest that brain cells work together to first provide a protective response that becomes damaging in the later stages of the disease. The results may help to determine the optimal window of opportunity to therapeutically intervene during disease progression.
One of the hallmarks of Alzheimer’s disease is the accumulation of the protein amyloid beta (Aβ) into plaques around neurons. Genetic evidence suggests that Aβ may play a key role in the development of the condition and the eventual death of neurons in the brain. Decades of research have sought to uncover the precise events occurring, though many questions still remain. In this latest study, published today (22 July) in Cell, researchers turned to advanced technology in ‘spatial transcriptomics’ to decode the molecular changes in cells near to the Aβ plaques.
Performing analysis within a 100-micrometre perimeter – around the diameter of an average human hair – of the plaques, the researchers discovered that the cells were producing Ribonucleic Acid (RNA), instructions for making proteins, in two broad profiles during the course of the disease.
In what would be the early or mild stages of Aβ build-up in the mouse model of disease, the team found that genes were enriched in a particular cell in the brain, oligodendrocytes, whose main role involves supporting neurons with the rapid transmission of electrical signals. The team speculate that this profile may be protective, possibly involving repair to the brain.
However, as the Aβ continues to accumulate, the researchers observed a different profile of gene expression involving 57 plaque-induced genes (PIGs) from several support cells such as microglia and astrocytes. This profile was associated with increased inflammation and oxidative stress, perhaps indicating that cells in the brain were being overwhelmed by the increasing Aβ plaques. Dr Mark Fiers, co-lead of the study, noted that many of the genes in both these profiles or networks were similarly altered in the human post-mortem brain tissue, supporting clinical relevance from the mouse model of disease.
Commenting on the study, Prof Bart De Strooper, Group Leader at VIB-KU Leuven and UK DRI Director, said:
“Our data demonstrate that amyloid plaques are not innocent bystanders of the disease, as has been sometimes suggested, but in fact induce a strong and coordinated response of all surrounding cell types.”
“Further work is needed to understand whether, and when, removal of amyloid plaques—for instance by antibody therapy currently in development to treat amyloid plaques—is sufficient to reverse these ongoing cellular processes.”
The team hope that, in addition to the knowledge gained in this study on cellular response in Alzheimer’s disease, spatial transcriptomics can be used widely across dementia research to uncover molecular changes occurring in other neurodegenerative diseases, helping identify novel drug targets for therapeutics.
Reference
Image courtesy of the De Strooper Lab -Section of half a mouse brain in which different types of brain cells are stained
Article published: 22 July 2020