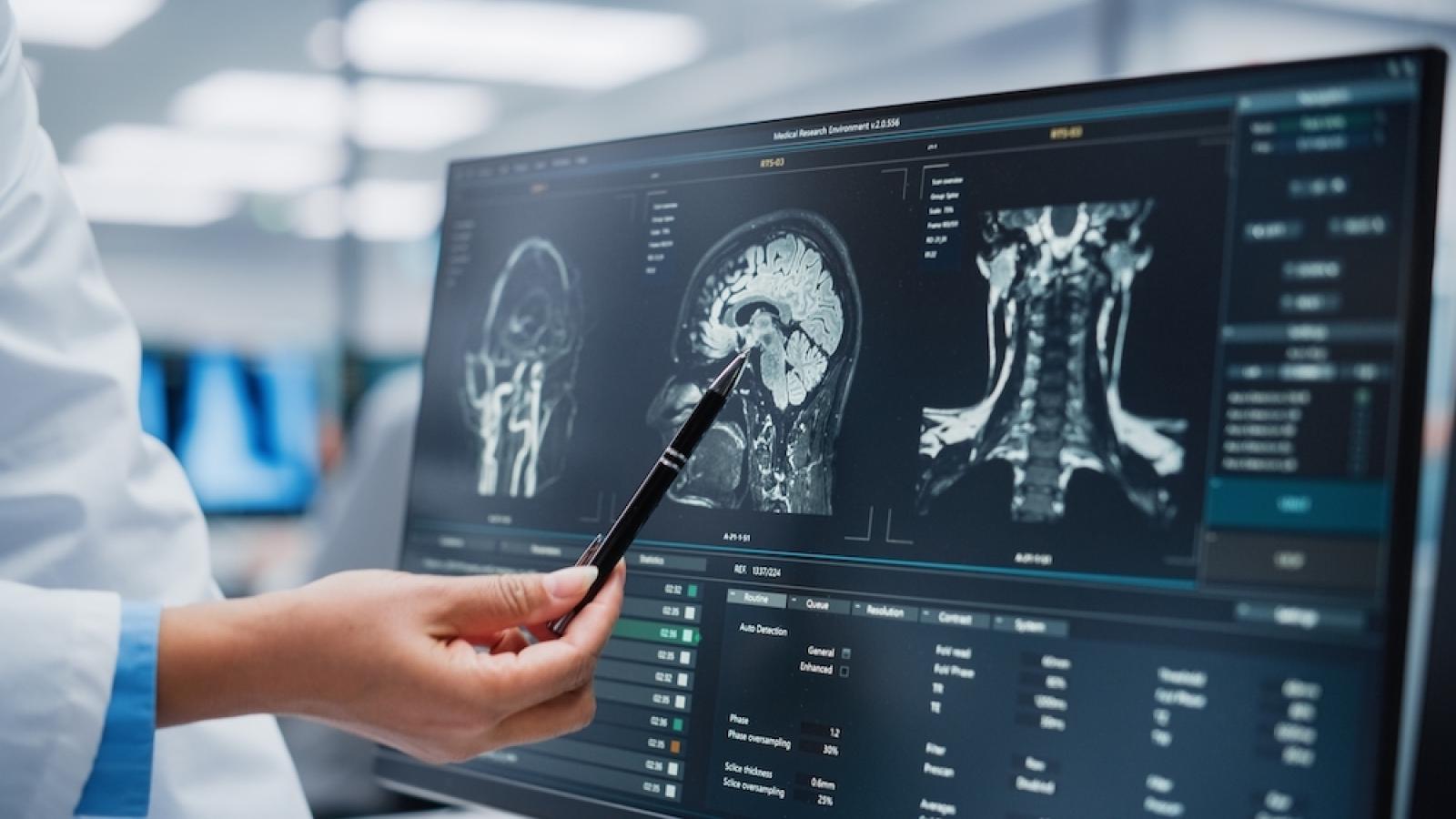
Alzheimer’s research is gaining ground. Recently, two new drugs - lecanemab and donanemab - have proven to significantly slow the spread of the disease in clinical trials. Both drugs work by removing amyloid, a toxic protein in the brain linked with Alzheimer’s.
These drugs are disease-modifying therapies, meaning that they tackle the root causes of Alzheimer’s itself - as opposed to current treatments approved for use in the UK, which only manage disease symptoms. The success of these drugs in trials has brought hope, and this has been hailed as a turning point in dementia research.
Despite this breakthrough, much progress still needs to be made. Although lecanemab and donanemab slow the disease, there is debate over whether these effects can significantly impact the life of somebody with Alzheimer’s, especially when weighed up against the drugs’ potential side effects. Additionally, the drugs work less effectively in some people than others. These limitations likely arise as amyloid is only one part of the picture: there are many other factors in the brain and body that contribute to Alzheimer’s.
Targeting these other factors alongside amyloid may lead to a more comprehensive and effective therapy programme. In this article, we will discuss some of these promising targets.
Tau: another hallmark disease protein
Amyloid isn’t the only toxic protein linked with Alzheimer’s: another protein called tau is also responsible. Normally, tau helps to maintain the structure of neurons in the brain. However, in Alzheimer’s, an abnormal form of tau, which disrupts neuron structure, accumulates instead. Compared to amyloid, the tau protein correlates more strongly with neurodegeneration.
This means that targeting tau can lead to extremely impactful treatments for Alzheimer’s - and it’s a major target being looked at by researchers. Of all Alzheimer’s drugs that are currently undergoing clinical trials, tau-targeting drugs make up a significant portion. These drugs include antibodies designed to attack the tau protein itself, drugs that stop tau proteins from clustering together, drugs that stop tau from being produced in the brain, and more.
As of now, two tau-targeting drugs are in Phase 3, the final stage of clinical trials. These drugs are E2814, an anti-tau antibody; and LMTM, which prevents tau proteins from clustering and dissolves existing clusters. Eight more drugs targeting tau are currently in Phase 2 clinical trials.
Research led by UK DRI scientist Dr Will McEwan has found a way to optimise future antibody therapies targeting tau. Dr McEwan discovered that a certain immune mechanism in the brain cells deteriorates tau proteins, while avoiding negative inflammatory side effects. Future treatments harnessing this mechanism could be much safer while still effective.
new drugs have proven to slow the spread of Alzheimer's in trials - but there are many other promising targets also being investigated
Targeting the immune system
Another factor that can contribute to Alzheimer’s is harmful changes associated with the immune system. During the development of the disease, the body’s immune system attempts to clear out the toxic proteins that begin to build up. As part of this, inflammation is triggered in the brain. While some inflammation is good, if it is a large or persistent response, healthy brain cells can become damaged, inadvertently contributing further to the progression of Alzheimer’s.
By preventing an extreme or chronic response, targeting inflammation could significantly improve outcomes for people with Alzheimer’s. Inflammation is one of the most examined targets, with drugs targeting inflammation making up almost one-fifth of all disease-modifying Alzheimer’s therapies currently in clinical trials.
Creating treatments that target inflammation can be particularly tricky. Because inflammation is a part of the body’s natural immune response, targeting it in the wrong way, or at the wrong time or place, could negatively interfere with the immune system’s fight against amyloid. However, scientists are starting to successfully navigate these challenges, and research has been strongly progressing: so far, two anti-inflammatory drugs have made it to Phase 3 of clinical trials.
One notable treatment in the works is a repurposed form of the existing drug semaglutide. Originally intended to target inflammation in diabetes, researchers have repurposed it to treat inflammation in Alzheimer’s disease. It’s currently in Phase 3 of clinical trials, and results are expected in 2025.
Aside from inflammation-causing factors, other parts of the immune system can make effective targets as well. A recent study led by Dr Soyon Hong and Dr Sebastiaan de Schepper found that a certain immune protein causes connections between neurons to degrade, and may lead to substantial improvement if targeted.
Blood vessels: a new avenue showing plenty of promise
The role of the brain’s vasculature in the development of Alzheimer’s has been previously overlooked. However, evidence suggests that blood flow to the brain is decreased in the disease, resulting in this energy-hungry organ being starved of oxygen and subsequent cognitive decline.
Research has also found that the blood-brain barrier – the highly restrictive structure at the interface between the blood and brain - becomes dysfunctional in Alzheimer’s. This means that more substances from the blood can gain access to the brain, including immune components which can drive harmful neuroinflammation. Blood-brain barrier dysfunction occurs at an early stage of Alzheimer’s - so studying this can be especially valuable as it can help us treat Alzheimer’s before significant damage occurs.
Vascular health issues and Alzheimer’s are also very intertwined. Alzheimer’s disease and vascular dementia is the most common type of mixed dementia, and conditions which damage the blood vessels increase the risk of developing Alzheimer’s. To ensure the best outcomes for people affected by Alzheimer’s, these issues should be considered – making it all the more important to target blood vessels when creating treatments.
Treatments in the works that target blood vessels include a combination of the drugs telmisartan and perindopril, which expands the vessels to make more room for blood; and Yangxue Qingnao pills, which promote the flow of blood itself. Because it’s a newer area being looked at, drugs targeting blood vessels are still in the early stages: they’ve only made it to Phase 2 of clinical trials so far. However, this area is rapidly growing in attention, with increasing interest and investment into vascular research.
UK DRI researcher Prof Joanna Wardlaw CBE is also paving the way in drug research. She recently led a successful clinical trial testing two existing drugs, which were repurposed to treat strokes in cerebral small vessel disease (cSVD). cSVD often co-occurs with Alzheimer’s disease, and evidence suggests that the pathologies of both are interlinked – indicating that the drugs might help to alleviate Alzheimer’s as well.
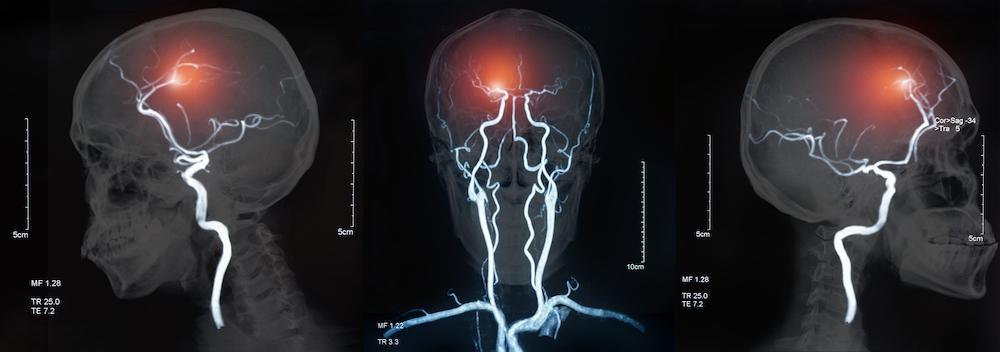
targeting the blood vessels in the brain are undergoing clinical trials
Making these treatments a reality
These potential targets are just a few examples - there’s also many others undergoing thorough study by researchers. Having a wide variety of targets is vital: since so many factors are linked to Alzheimer’s, its unlikely that a sole therapy will fully treat the disease. There’s no silver bullet for Alzheimer’s disease - overcoming it will only happen through a variety of therapies working together.
Many targets are also important because different people are at higher risk of different Alzheimer’s-inducing factors, and certain factors can also have a larger effect at different stages of the disease. Having available therapies that cover various factors means that people can receive treatments that work best for them.
But to make these treatments a reality, more support for fundamental discovery research is needed. Dementia research has been historically under-funded and under-resourced; as a result, there are still gaps in our knowledge of the condition. To fill these gaps, we need more investment into research on the biological mechanisms behind dementia, which will help us find more feasible and effective ways of targeting underlying factors.
But there’s still hope that we’ll get there – with the arrival of lecanemab and donanemab, there has been renewed interest and enthusiasm for neuroscience research that can help us learn more about Alzheimer’s and how to tackle it. And with increased support and collaboration, we’ll be able to create fully effective treatments that will help all affected by Alzheimer’s.
This World Alzheimer’s Month, the UK DRI is highlighting how its research in various fields is filling the dementia knowledge gap and bringing us closer to effective treatments for all. We’re also bringing attention to what still needs to be done, trying to raise support for dementia research overall. To learn more and join in the conversation, follow our campaign on social media using the hashtag #FillingTheGaps.
Article published: 14 September 2023
Image credits: Shutterstock