Studying the genetics of Alzheimer's disease
Researchers have found strong evidence that Alzheimer's disease develops through multiple genetic factors working together, rather than a single cause. While researchers have identified 75 different genetic regions linked to Alzheimer's risk, most don't have a clear standout variant that causes the disease. Importantly, many of these genetic risk factors are mainly active in brain immune cells called microglia, with some only found in these cells.
To better understand this complexity, the Williams Lab are developing special stem cell models from people who have either very high or very low numbers of these genetic risk factors. These models will help reveal how common forms of Alzheimer's develop, and are particularly valuable because it allows scientists to study how multiple genetic factors interact together, rather than looking at them in isolation. This research could open new avenues for preventing or treating Alzheimer's by providing a more complete picture of how the disease develops at a genetic level.
Latest news


Prof Julie Williams
Prof Julie Williams is a Group Leader at the UK DRI at Cardiff. Find out more about her career and expertise on her profile page.

Research summary
Deciphering genetic risk in Alzheimer's disease
The genetic evidence shows compelling evidence that Alzheimer’s (AD) is a disease of multiple components that combine to trigger and/or underpin disease development. The activity of disease components may differ between tissue types, with microglia strongly implicated. In addition, the majority of the 75 susceptibility loci which show genome-wide significance, do not present an obvious variant which shows association with disease above all others at the loci. Those few which do are more easily modelled and are already informing disease mechanisms. Thus, modelling specific risk factors and the complexity of combined risk factors are major challenges for the field. We also note that gene expression studies show the majority of genetic risk variants for AD are predominantly expressed in microglia, with some exclusively expressed in these cells.
We contend that induced pluripotent stem cell (iPSC) models derived from humans whose genomes possess an unusually high or low number of these risk loci, will provide novel models of the common forms of AD, capturing all risk variants in combinations observed in those with the common form of AD, will create valuable insights into disease biology and mechanisms, identify novel therapeutic targets, facilitate drug validation, and thus contribute to new treatments to prevent or modify AD.
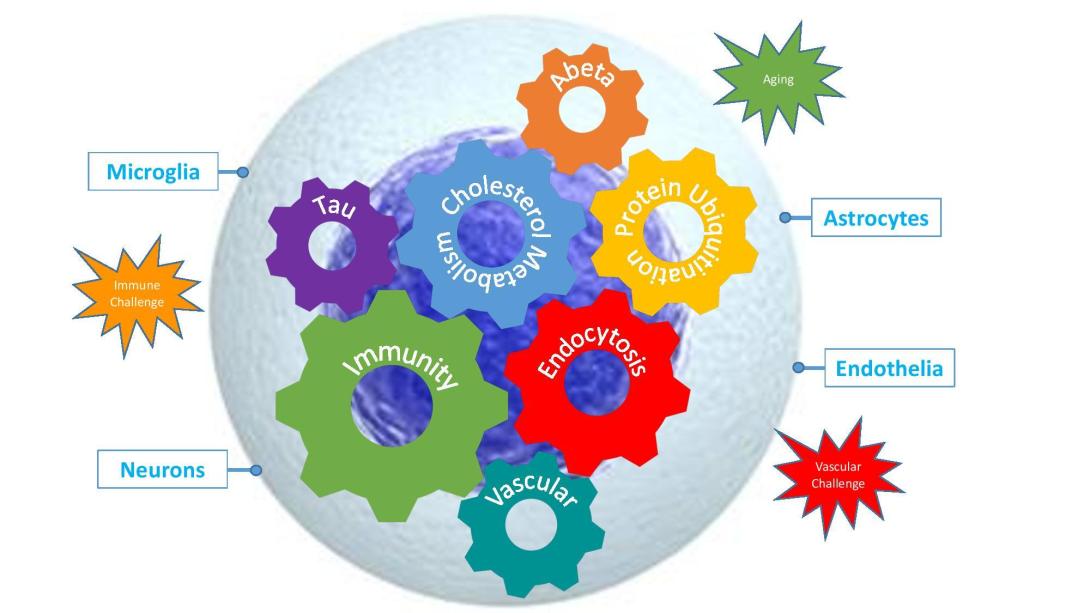
Multiplex Model: Depicting the biological processes (cogs) known to harbour genetic risk for Alzheimer's Disease, the disease specific cell types in which they act (blue boxes) and the environmental cues (explosions) that influence disease. Credit: Williams/Sims
Projects
Lab members
- Dr Natalia Connor-Robson (UK DRI Emerging Leader)
- Dr Gaynor Smith (Lecturer and Momentum award holder)
- Dr Owen Peters (Lecturer and Momentum award holder)
- Dr Atahualpa Castillo Morales (Postdoctoral Researcher)
- Dr Hazel-Hall Roberts (Postdoctoral Researcher)
- Dr Kimberly Jones (Postdoctoral Researcher)
- Dr Patricia Dos Santos Rodrigues (Postdoctoral Researcher)
- Rachel O'Donoghue (Research Assistant)
- Dr Rebecca Sims (Senior Researcher)
- Dr Samuel Braithwaite (Infrastructure Manager)
- Dr Rebecca Mahoney (Bioinfomatician)
- Srilakshmi Goberdhan (Senior Technician)
- Kristen Baille (Technician)
- Jincy Winston (Technician)
- Rebekah Cooke (PhD student)
- Pete Greer (PhD student)
- Eilish Mackinnon (PhD student)
- Johanna Maninger (PhD student)
- Karolina Nowak (PhD student)
- Elisa Salis (PhD student)
- Lucie Tkacova (PhD student)
- Izzy Gorman (Student)
- Andrew Marshall (Student)
Lab funders
Thank you to all those who support the Williams Lab!