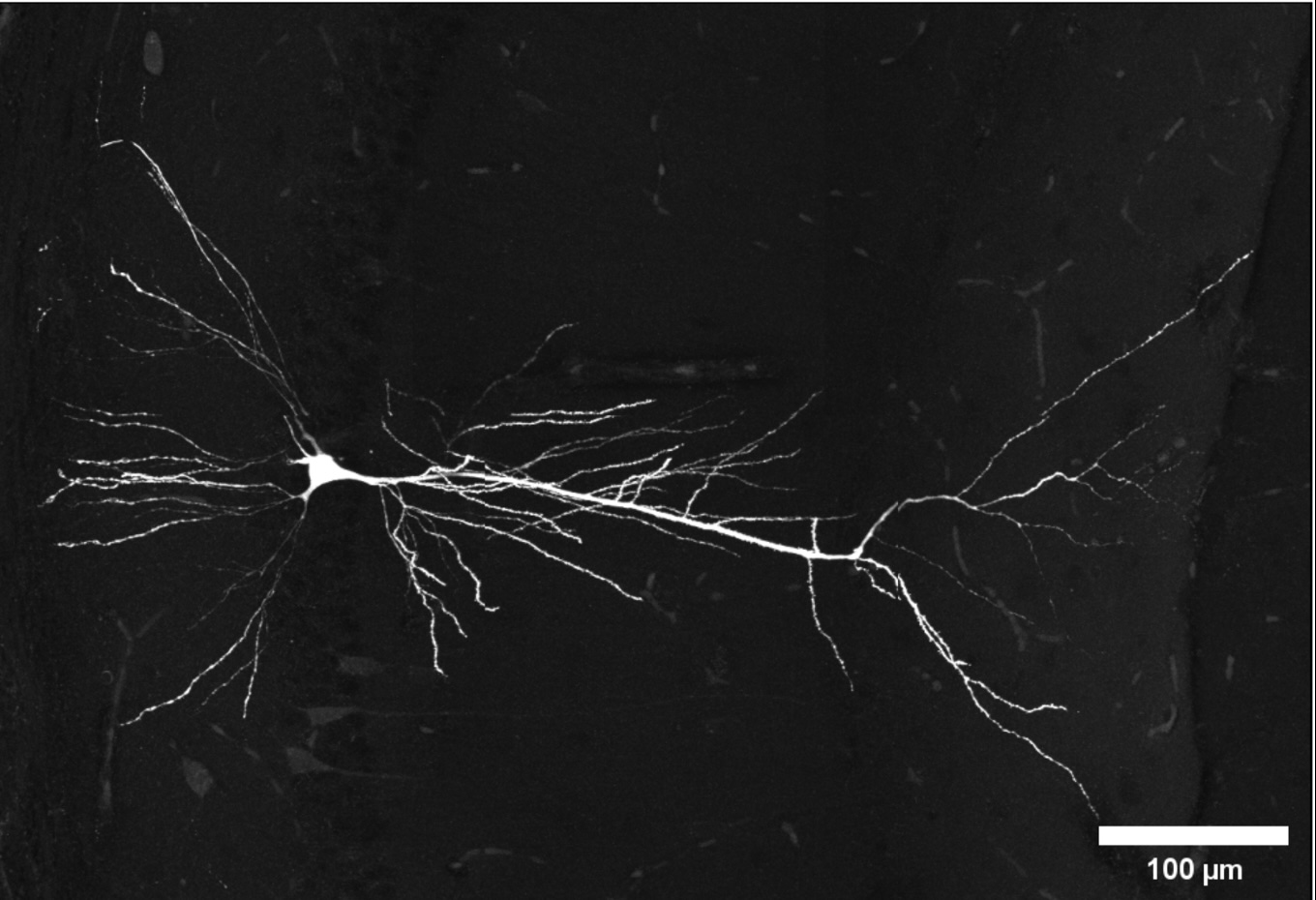
Biography
Dr Jian Gan is a cellular/systems neuroscientist. After obtaining his PhD from the University of Glasgow, supported by a Synergy Postgraduate Award and an Overseas Research Student Award, he went on to work in the lab of Prof. Peter Jonas at the University of Freiburg, Germany and later Institute of Science and Technology Austria near Vienna, where he employed and optimised in vivo high-resolution patch-clamp recording technique to elucidate synaptic mechanism of hippocampal oscillations that are essential for spatial coding and memory consolidation. He went on to gain further experience in cognitive and behavioural neurobiology using high-density population recording and virtual reality in the lab of Prof. Thomas Klausberger at the Medical University of Vienna, before setting up his own lab at the University of Edinburgh.
News
Key publications
Gan Lab
Explore the work of the Gan Lab, aiming to understand why and how neurons and brain circuits become dysfunctional in vivo