Biography
An expert in the innate immune system, Prof Phil Taylor leads an exciting UK DRI research programme focused on rare coding variants in Alzheimer's disease. Phil's background lies in studying Human Genetics at UCL, obtaining a PhD in Molecular Genetics from Imperial College London. He began intensive study of macrophages at Oxford University in the late nineties before arriving at Cardiff University in 2006. Throughout his career Phil has been recipient of a number of distinguished funding awards, and brings diverse experience to the UK DRI, including an interest in promoting the 3Rs - a framework for performing more humane animal research.
News

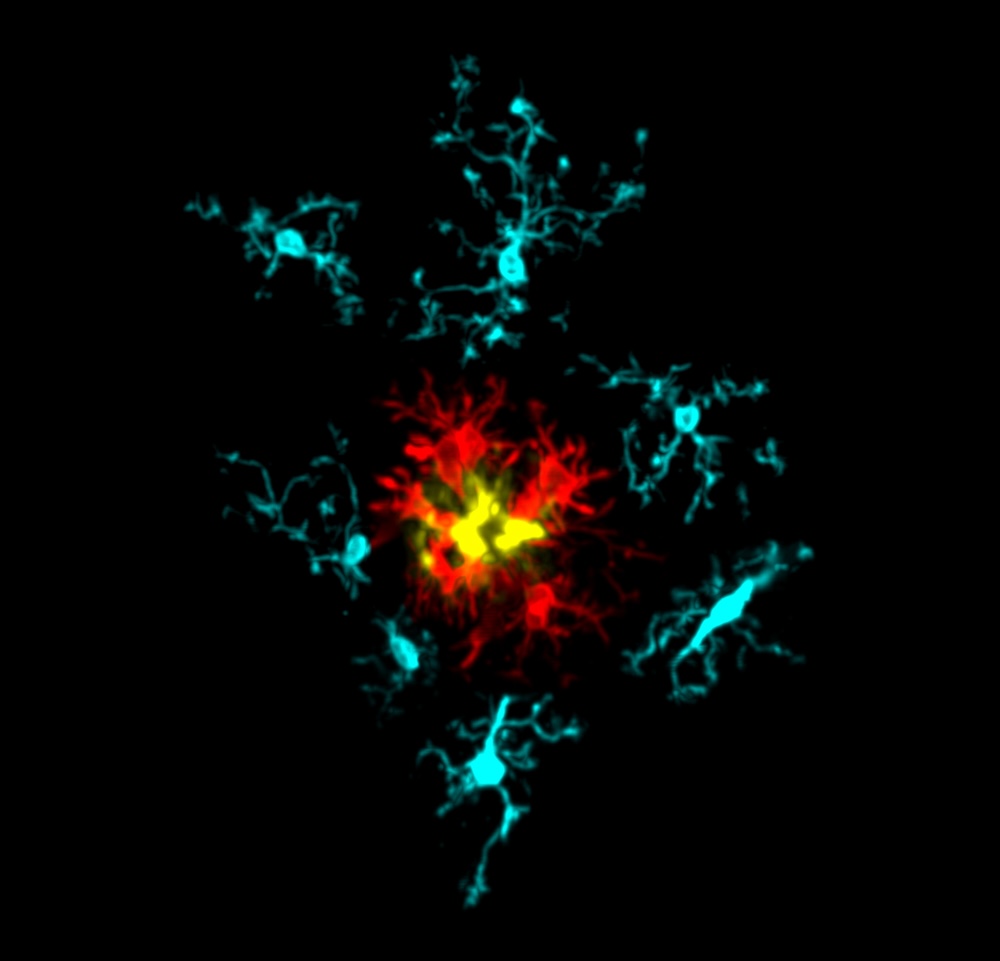
Taylor Lab
Explore the work of the Taylor Lab, dissecting the role of the immune system in Alzheimer's disease