Dissecting the role of the immune system in Alzheimer's disease
Alzheimer’s disease is a progressive brain disease that causes dementia. The most common form occurs in people who are age 65 and older, but there are also early-onset forms that can affect younger people.
While we know that early-onset Alzheimer’s disease is often caused by faults in high-risk genes, the role of genetics is less clear cut for late-onset forms of the condition. Using a relatively new approach called genome-wide association studies, researchers have identified more than 20 gene variations that can influence a person's risk – in combination with lifestyle and environmental factors. Many of the most recently-identified genetic risk factors have roles in the immune system, firmly pointing the finger for a role for microglia – a specialised type of immune cell that exists within the brain.
The Taylor Lab is exploring the biological consequences of gene variants known to influence a person’s risk of developing Alzheimer’s disease to dissect how microglia may contribute to disease development. The results could lead to powerful new treatments that can slow down, stop or reverse disease progression. As the immune system is also implicated Huntington’s and Parkinson’s diseases, any new discoveries or treatments could also benefit people with other neurodegenerative conditions.
Latest news

Prof Phil Taylor
Prof Phil Taylor is a Group Leader at UK DRI at Cardiff. Find out more about his career and expertise on his profile page.

Research summary
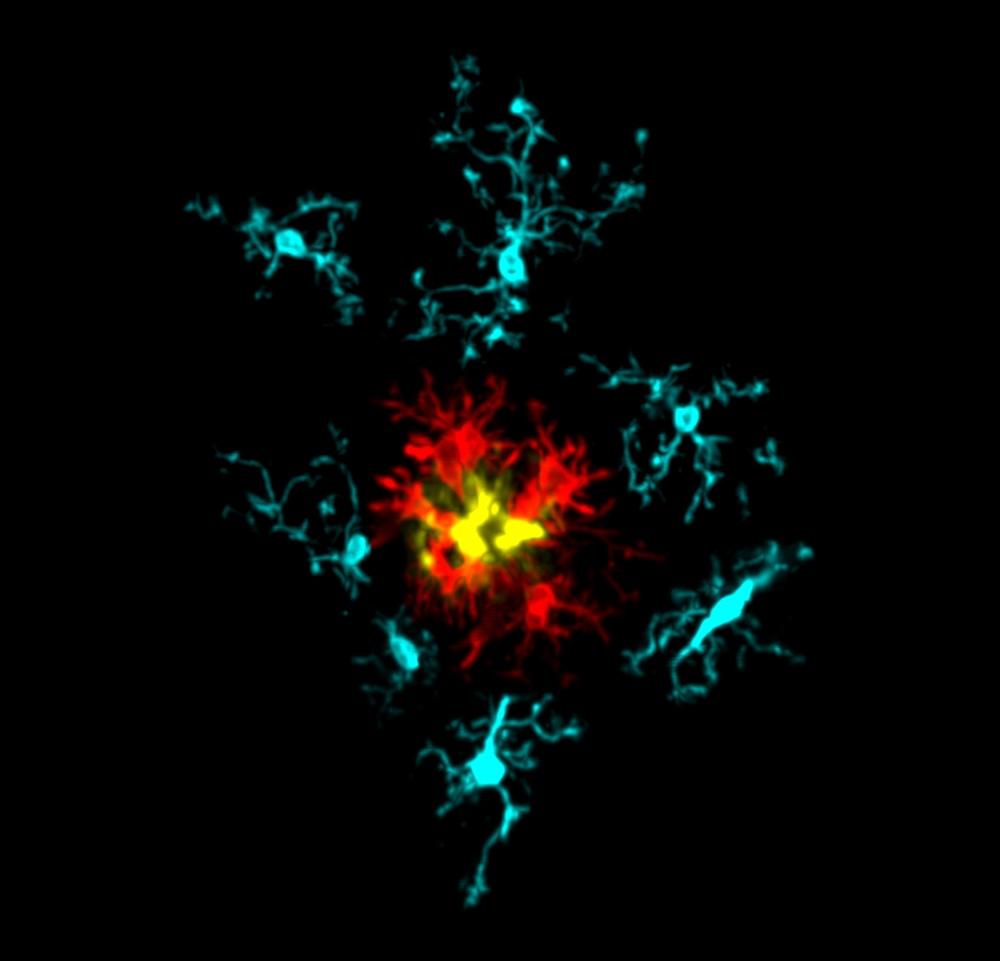
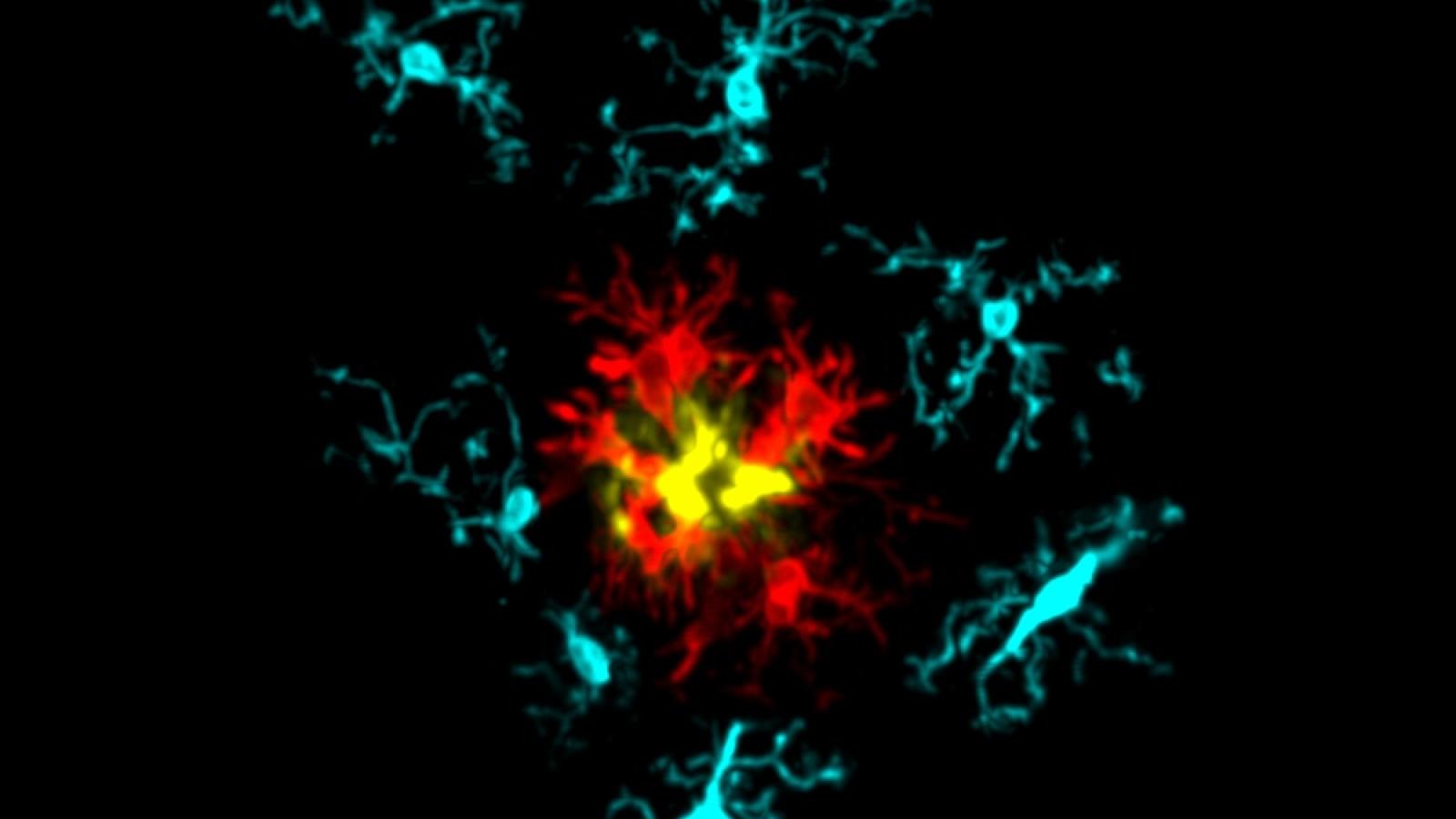
This image exemplifies the microglial response to amyloid pathology in the AppN-G-F mouse model of Alzheimer’s disease. It is an immunofluorescence confocal image showing a single amyloid plaque stained with X34 (yellow), surrounded by IBA1+ microglia, which have been ‘isolated’ using Imaris 3D software. The microglia are pseudo-coloured based on the relative presence or absence of the DAM marker CLEC7A (red) and the homeostatic marker TMEM119 (cyan). Understanding the mechanistic changes in microglia and their response patterns will provide opportunities for discovering therapeutic strategies for irreversible neurodegenerative pathologies. Credit: Ryan Bevan, Taylor Lab
Towards an in vivo functional dissection of the role of microglia and macrophages in Alzheimer's disease aetiology
Novel genetic findings from the International Genomics of Alzheimer’s Project (IGAP) consortium implicate genes and pathways not previously known to be associated with dementia. This has firmly established genes of the immune system in dementia aetiology and indicates microglia/macrophages (MØ) are important in the development of Alzheimer’s disease (AD).
Microglia are tissue-resident (Res) MØ considered to play fundamental roles in synaptic pruning and amyloid beta clearance. Furthermore, additional epigenetic analysis carried out in Cardiff suggests that disease-associated variants are concentrated in specific transcriptional networks. Results indicate that SPI1 and MEF2 transcriptional networks are central to AD risk mechanisms. SPI1 is a central transcription factor in the microglial activation state, has a significant association with AD and shows AD-associated variants altering expression in monocytes in the direction predicted by biology. A common haplotype associated with reduced SPI1 expression is associated with later onset of Alzheimer’s disease. In addition, several rare coding variants, for example in PLCG2 and ABI3, are associated with AD, and lie in the network controlled by SPI1, along with TREM2 and CSF1R. In the case of PLCG2, the rare variant (P522R) is AD protective.
The identification of specific DNA variants, particularly coding variants, in AD provides an opportunity to dissect immune mechanisms underlying disease. Notably, immune networks are implicated in Huntington’s and Parkinson’s diseases, suggesting that these diseases have some parallel immune pathway pathology and might benefit from similar treatments. Microglia are specialised Res MØ and the recent work of Prof Philip Taylor and his team has demonstrated that MØ phenotype is dictated by the tissue context. The in vivo study of microglia/MØ function is therefore essential to understand how alterations in basic physiological processes, mostly implicated by the genetics of disease, contribute to dysfunctions that may predispose to disease.
Main objectives and research goals:
The objective of this programme of work is to establish a functional pipeline for the study of disease-associated genes and polymorphisms in microglia and MØ in the context of dementia-related disease in vivo. The team are initially focussing on the impact of coding mutations in PLCG2 and ABI3, with newly-identified variants feeding future studies. Their aims are to determine:
- The role of rare coding variants in microglial and MØ development and renewal, and in cellular activation.
- The quantitative impact of rare coding variants function and whether these are selective on myeloid cell compartments or at specific developmental stages.
- An objective and global view by transcriptomic analysis of the impact of variants on cellular activation and potential key mechanistic pathways by functional genomic approaches.
- The impact of variants on the development of AD.
Key publications
Vacancies
Lab members
- Dr Magdalena Czubala (Postdoctoral Researcher)
- Dr Emily Maguire (Postdoctoral Researcher)
- Dr Carolina Toste (Postdoctoral Researcher)
- Dr Ryan Bevan (Postdoctoral Researcher)
- Dr Rachel Hills (Postdoctoral Researcher)
- Dr Charlotte Bridge (Postdoctoral Researcher)
- Dr Ana Cardus-Figueras (Research Associate)
- Alana Jones (PhD Student)
- Ed Birt (PhD Student)
- Alice Smith (PTY Student)
Collaborators







Lab funders
Thank you to all those who support the Taylor Lab!