Biography
Having graduated with degrees in mathematics and psychology from the University of Freiburg, Dr Sarah Marzi completed her PhD in complex disease epigenetics with Prof Jonathan Mill at King’s College London and worked as a postdoctoral researcher with Prof Vardhman Rakyan at Queen Mary University of London. At the end of 2019, she established an independent research group as an Edmond and Lily Safra Research Fellow, joining the UK DRI at Imperial. The Marzi lab combines experimental genomic and epigenomic techniques with innovative statistical and computational analyses to understand gene regulatory mechanisms contributing to the earliest stages of neurodegenerative disease. Dr Marzi became a UK DRI Emerging Leader at UK DRI at Imperial in 2021, and a Group Leader at the UK DRI at King's in 2023.
News
Key publications
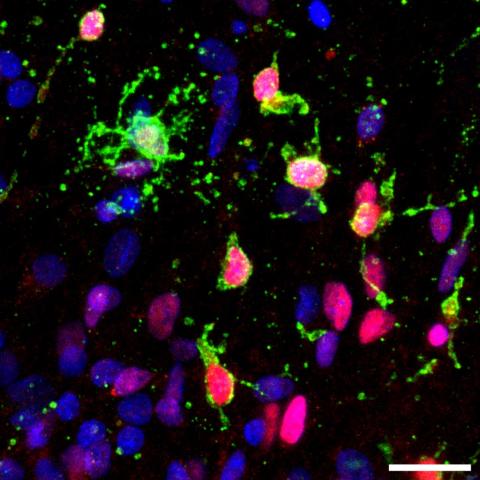
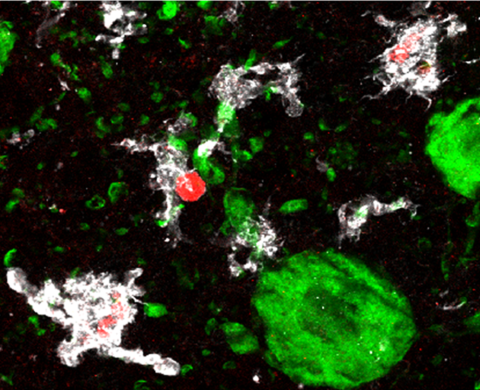
Marzi Lab
Explore the work of the Marzi Lab, focusing on finding out how epigenetics regulates biological mechanisms in health and during disease.