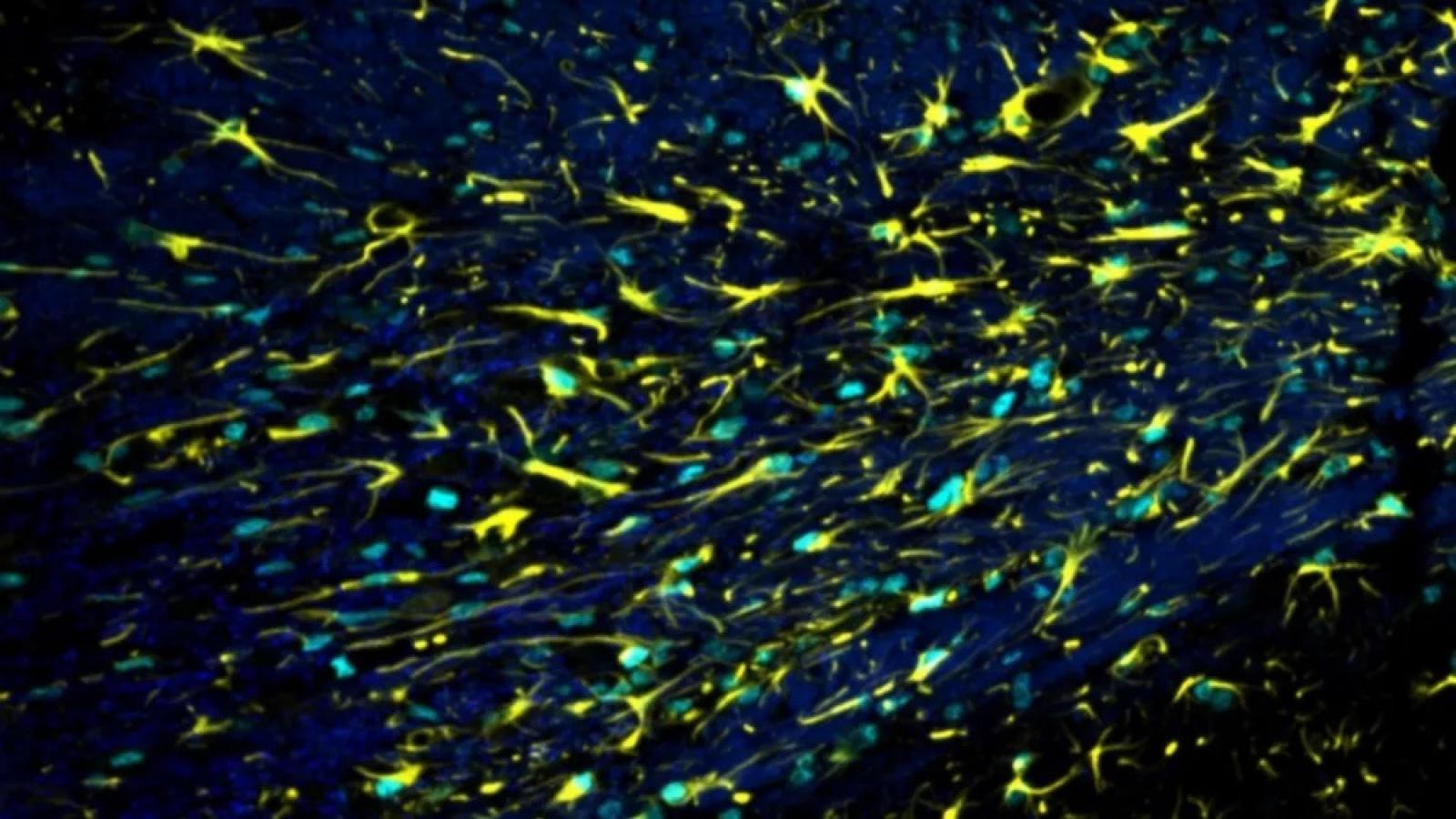
Key details
Understanding how brain cell insulation changes with ageing and dementia
Cognitive decline, defined as a decrease in thinking and memory ability, occurs with normal ageing and in neurodegenerative diseases like Alzheimer’s. The lack of therapies that can halt cognitive decline highlights the importance of discovering the cells and molecules involved in this process.
Thinking and memory have recently been shown to be controlled by myelin, the insulation surrounding nerve fibres which is key for nerve health and function. With ageing and in Alzheimer’s disease, myelin becomes unhealthy. This suggests that myelin might be dysfunctional in ageing and dementia, and is therefore an important area of research to develop new and effective drugs.
The Miron Lab is using a combination of human brain tissue analysis and experimental modelling to understand how myelin changes with aging and dementia, and how we can intervene to prevent cognitive decline.
Latest news

Prof Veronique Miron
Prof Veronique Miron is a Group Leader at the UK DRI at Edinburgh. Find out more about her career and expertise on her profile page.

Research summary
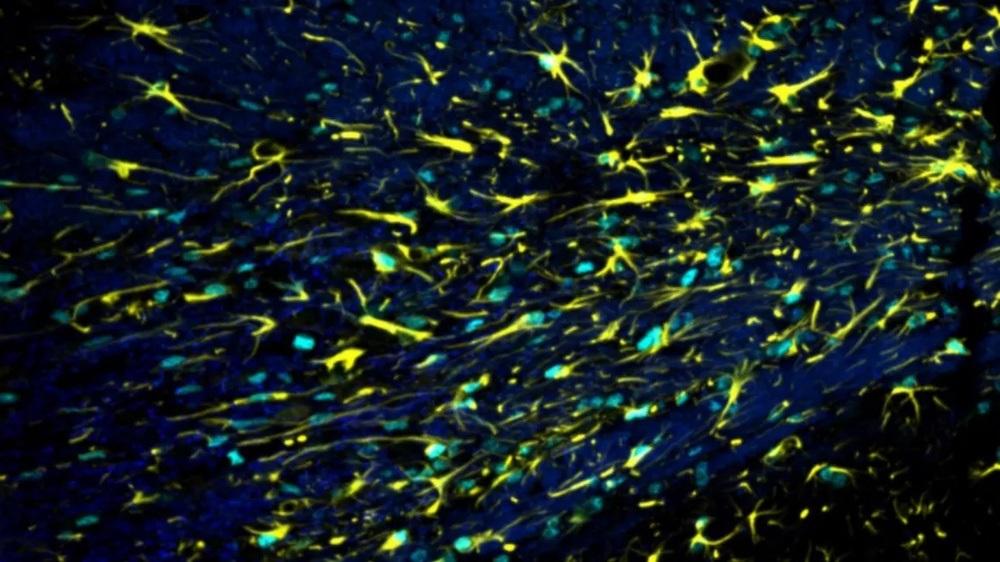
Astrocyte cells. Credit: Miron Lab
How does myelin integrity change to regulate cognitive function in ageing and dementia?
Cognitive decline with ageing and neurodegenerative disease is a growing socioeconomic burden for which we lack effective therapies. In the past decade, new research has revealed that healthy cognition requires healthy myelin – the membrane ensheathing axons which provides metabolic and trophic support. Accordingly, frequency of white matter abnormalities are predictive of degree of cognitive decline in aged individuals. However, the cellular and molecular mechanisms underpinning these abnormalities, and how they contribute to cognitive decline, are unclear.
The Miron Lab is using a combination of human neuropathology, transgenic mouse models, spatial transcriptomics and single cell RNA sequencing, and ultrastructural myelin analyses to understand how myelin, and the cells that make myelin (oligodendrocytes), are dysfunctional with ageing and in Alzheimer’s disease.
The team have uncovered that interactions between glial cells and oligodendrocytes is critical for the maintenance of myelin with ageing, and its repair following damage. They revealed that central nervous system macrophages restrict oligodendrocyte heterogeneity to prevent myelin abnormalities and damage (Nature 2023), and support myelin repair (Nature Neuroscience 2019, Acta Neuropathologica 2018, Nature Neuroscience 2013). They also found a protective role for astrocytes in supporting oligodendrocyte survival during this repair (Nature Communications 2023).
The Miron Lab will build on these findings to understand:
- How oligodendrocytes and myelin become dysfunctional to drive cognitive decline with aging
- How oligodendrocytes and myelin become dysfunctional to cause neurodegeneration in Alzheimer’s disease
- How interactions between oligodendrocytes and glial cells regulates their dysregulation in aging and disease
Key publications
Vacancies
Lab members
- Ayisha Mahmood (Postdoctoral Researcher)
- Alana Hoffmann (Postdoctoral Researcher)
- Christina Brown (Postdoctoral Researcher)
- Georgie Craig (Postdoctoral Researcher)
- Jamie Rose (Lab Manager/Senior Technician)
- Lucy Ryan (PhD Student)
Collaborators









Lab funders
Thank you to all those who support the Miron Lab!