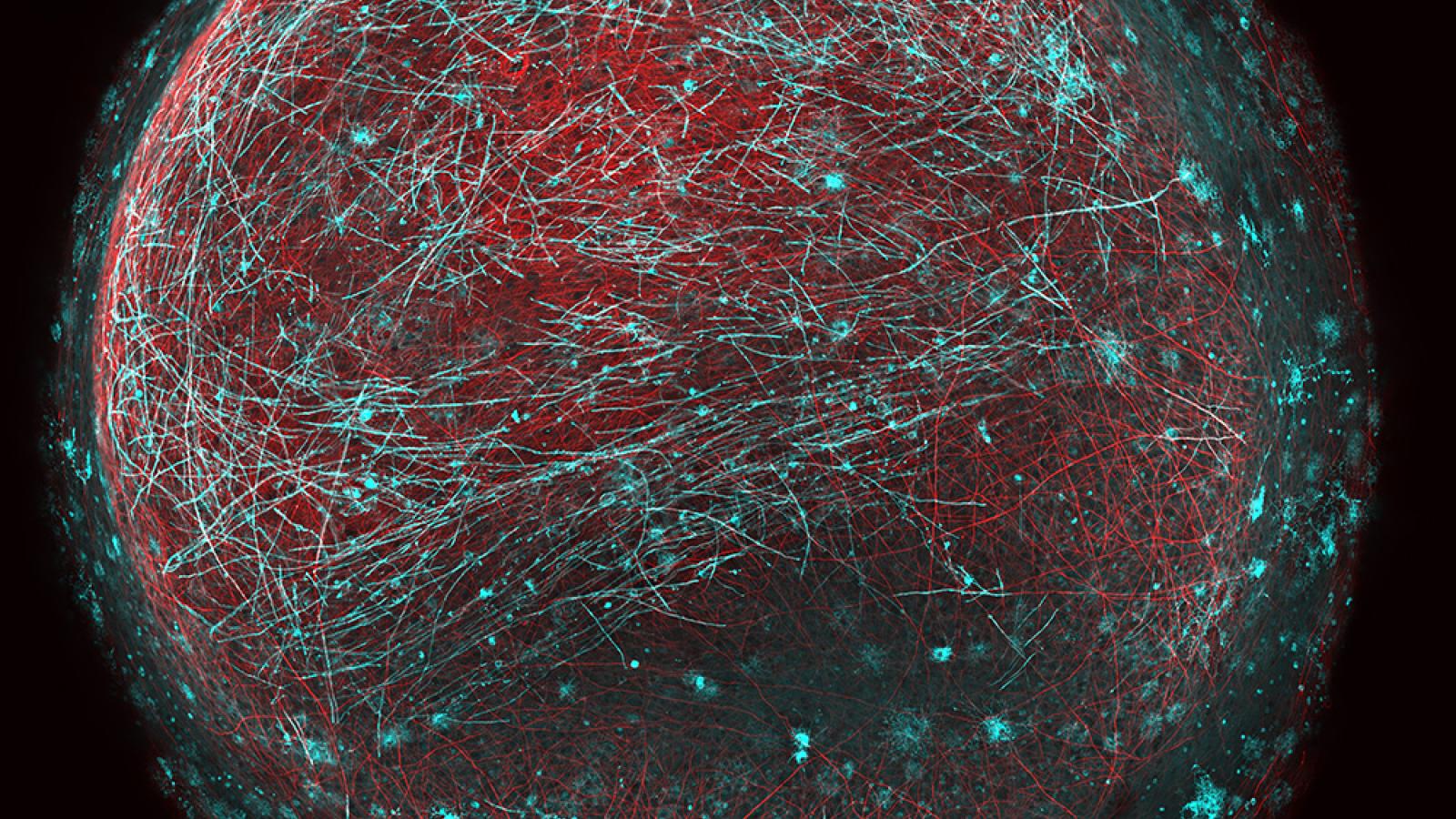
Understanding the underlying mechanisms that cause neurons to degenerate in ALS
Amyotrophic lateral sclerosis (ALS) , a type of motor neurone disease (MND), is a neurodegenerative disease that affects the brain and nerves, causing weakness that gets worse over time, and is eventually fatal. The Selvaraj Lab seeks to understand the underlying mechanisms that cause neurons to degenerate in ALS, with a view to identifying new therapeutic targets.
There is increasing evidence to suggest that the disease is caused by multiple factors, and that these affect different parts of brain cells. Therefore, a strategy against ALS needs to consider these multiple factors. The Selvaraj Lab has previously shown that dysfunction in a type of receptor in the brain, called a glutamate receptor, contributes to the development of C9ORF72 ALS, a form of familial, or genetic, ALS. How this occurs is not yet well understood, and whether the glutamate receptor dysfunction also occurs in sporadic ALS is currently unknown. This programme aims to investigate these points, and identify genes and pathways involved in glutamate receptor dysfunction that could potentially be targets for therapeutic intervention.
The neuromuscular junction (NMJ) is the point at which nerves connect to skeletal muscle, important for movement. In ALS, changes occur at the NMJ prior to the loss of nerve cells. The Selvaraj Lab aims to better understand how NMJs degenerate in ALS, to study how this progresses over time as the disease develops, and to determine how the NMJ is regulated, as this will enable the identification of new therapies for ALS.
Latest news


Dr Bhuvaneish T Selvaraj
Dr Bhuvaneish T Selvaraj is an Emerging Leader at the UK DRI at Edinburgh. Find out more about his career and expertise on his profile page.

Research summary
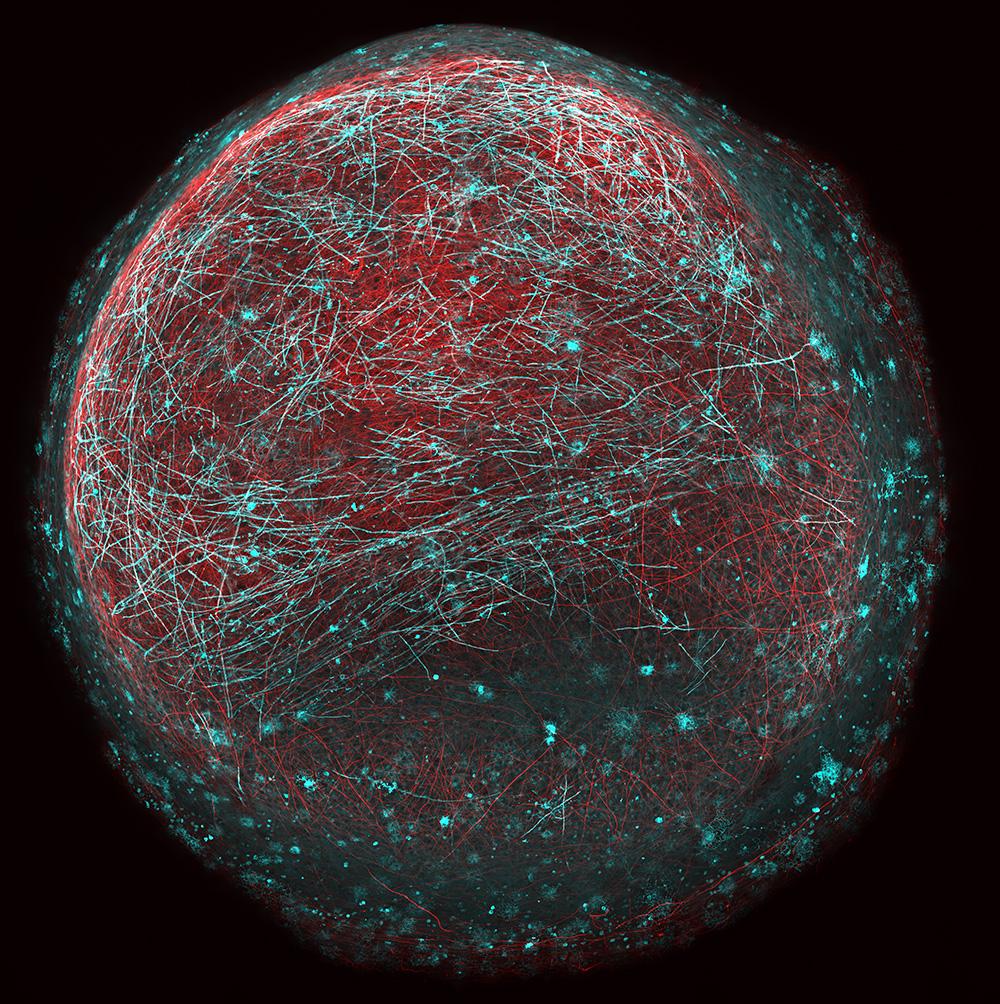
Human stem cell-derived 'mini brain' containing neurons (red) being wrapped in specialised 'myelin' insulation (blue). Each mini brain is 1-2mm in diameter. This model can be used study human myelin development and disease and to test for new drugs that promote myelin repair. Credit: Dr Owen Gwydion James, Chandran lab
Mechanisms of selective vulnerability of motor neurons in amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis is a fatal neurodegenerative disease caused by axonal degeneration and loss of motor neurons. The overall objective of Dr Selvaraj’s research programme is to understand mechanisms of selective vulnerability of motor neuron degeneration in ALS and translating that knowledge to identify novel therapeutic targets and strategies. To address this scientific question, Dr Selvaraj’s team uses human stem cell disease modelling including complex organoids and deeply phenotype and clinically linked patient autopsy samples combining with state-of-the art molecular interrogation tools to undertake mechanistic experiments.
Accumulating evidence suggest that MND is multifactorial, and independent pathomechanisms affecting distinct cellular compartments of motor neurons – soma and axon – leading to motor neuron loss and axon degeneration, respectively, thus a successful neuroprotective strategy in ALS would need to target both. The Selvaraj group has shown that glutamate receptor dysfunction, leading to increased vulnerability to excitotoxicity (a form of neuronal injury caused to excessive glutamatergic stimulation), contributes to disease pathogenesis of C9ORF72 ALS, the commonest form of genetic ALS. However, the molecular mechanism of these dysfunctions is still unclear and whether these dysfunctions are generalisable to sporadic ALS cases – which occurs in 90% of ALS cases, is unknown.
Therefore, the aim of this programme of research is to determine:
- Mechanistically how C9ORF72 mutation leads to glutamate receptor dysfunction and excitotoxicity
- Whether glutamate receptor dysfunction is generalisable to sporadic ALS - which causes 90% of ALS cases and how it mechanistically links to TDP43 pathology are observed in ca.97 % of ALS cases this includes genetic form of ALS and sporadic ALS with no known genetic mutations
- Finally, to perform genome wide CRISPR screening to identify genes/pathways that regulate glutamate receptor dysfunction which will enable the team to identify a rescue strategy for motor neuron vulnerability to excitotoxicity.
The neuromuscular junction (NMJ) is a chemical synapse between motor neuron and skeletal muscle which is crucial for muscle movement. In ALS, NMJ denervation followed by axonal degeneration is known to precede loss of motor neurons. Noting that human NMJs are distinct to other species both structurally and functionally, Dr Selvaraj’s team have developed a human stem cell-based neuro-muscular organoid model to study NMJ structure and function. They aim to better understand the mechanisms underlying how NMJs degenerate in ALS, to study temporal progression of the disease (MN loss and axon degeneration), determine local molecular factors that regulate NMJ maintenance, as this will enable us to identify novel therapies to limit or stop NMJ degeneration and, thus, slow disease progression in ALS.
Key publications
Vacancies
Lab members
- Dr Shreya Das Sharma (Postdoctoral Researcher)
- Dr Esra Ozkan (Postdoctoral Researcher)
- Dr Krishna Bhavsar (Postdoctoral Researcher)
- Dr Samantha Ho (Postdoctoral Researcher)
- Dr Mungo Harvey (Postdoctoral Researcher - jointly with Siddharthan Chandran)
- Dr Daria Iakovleva (Postdoctoral Researcher - jointly with Siddharthan Chandran)
- Dr Reiss Pal (Postdoctoral Researcher - jointly with Siddharthan Chandran)
- Aine Heffernen (PhD student)
- Jade Lucas (PhD Student)
- Irene Roig Ferrando (PhD Student)
Collaborators








Lab funders
Thank you to all those who support the Selvaraj Lab!