A team of scientists from the UK DRI at Cambridge and the Medical Research Council Laboratory of Molecular Biology (MRC LMB) have developed new therapies that selectively remove aggregated tau proteins, which are associated with Alzheimer’s disease, and improve symptoms of neurodegeneration in mice. The research is published in Cell and Science.
The team, co-led by UK DRI Group Leader Prof Will McEwan, demonstrate how utilising the unique capabilities of a protein called TRIM21 gives the potential therapies two key advantages. Firstly, they only destroy the disease-linked tau aggregates, leaving healthy tau proteins intact. And secondly, the therapies remove already established tau aggregates in mice, not just preventing their formation.
Trimming Alzheimer’s-linked proteins
Co-discovered by Prof Will McEwan during his postdoctoral studies, TRIM21 is a unique protein which is a key part of the immune response to viruses. Outside the cell, the body produces antibodies that bind to invading viruses. When the antibody-bound virus enters a cell, TRIM21 detects it and tags the virus as ‘garbage’, handing it over to the cell’s ‘garbage chute’, the proteasome, for destruction.
Last year, the researchers demonstrated that TRIM21 could be re-purposed to destroy tau protein aggregates associated with Alzheimer’s disease. By switching out antibodies that bind viruses for antibodies that bind to tau, TRIM21 was re-directed to send tau aggregates to be destroyed by the proteasome.
Tau aggregates are tucked away inside brain cells and very difficult to degrade. Critically, these new TRIM21-based therapies can be delivered directly inside cells, where the majority of tau aggregates reside.
Group Leader
New Trojan horse therapy for tau aggregates
In the new studies, the scientists used TRIM21 to create two new therapies to target tau aggregates.
The first therapy, ‘RING-nanobody’, combines a tau-binding nanobody – a miniature version of an antibody – with a part of the TRIM21 protein known as ‘RING’.
The second therapeutic, ‘RING-Bait’, has the TRIM21 RING joined to a copy of the tau protein itself. The RING-linked tau protein acts as bait – the aggregates incorporate it and TRIM21 RING gets incorporated as well. Once multiple RING-Baits are added to the aggregate, they become activated and cause the entire aggregate to be destroyed.
The researchers delivered the DNA encoding the TRIM21 therapies into cells containing aggregated tau and found it cleared the tau clumps. As hoped, normal tau was left undamaged.
Prof Will McEwan, co-leader of the studies, from the UK DRI at Cambridge said:
“Tau aggregates are tucked away inside brain cells and very difficult to degrade. Critically, these new TRIM21-based therapies can be delivered directly inside cells, where the majority of tau aggregates reside.
“We’ve found a way that not only degrades the tau aggregates, but leaves the healthy tau intact to do its job. The new strategy goes beyond what can be achieved with current antisense oligonucleotide (ASO) therapies that are being trialled, as it could avoid any potential long-term side-effects of eliminating normal tau.”
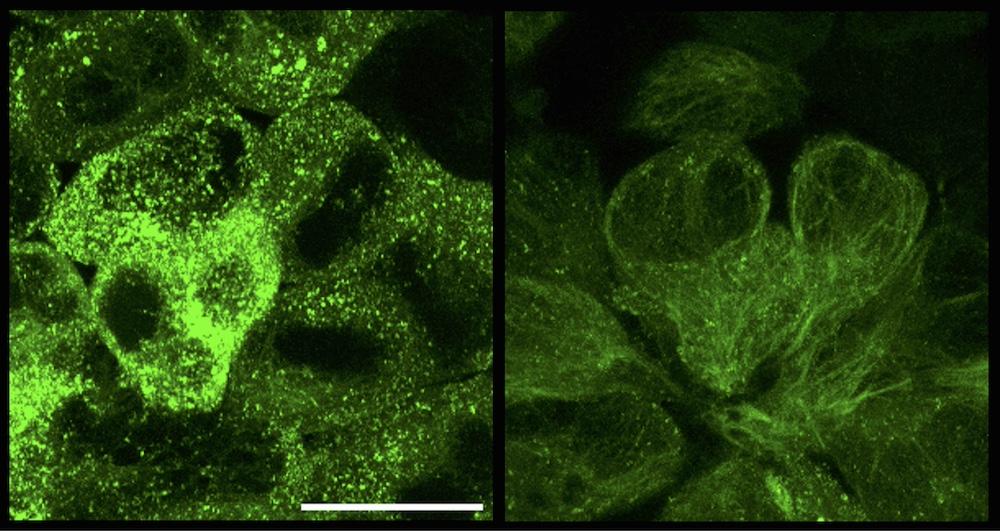
Cells containing tau aggregates (green) before (left) and 13 hours after treatment with RING-nanobody (right). Credit: Jonathan Benn
Since different neurodegenerative diseases can have different types of misfolded tau, they tested the therapies on cells containing aggregated tau proteins from brain tissue donated by people who had Alzheimer’s disease or progressive supranuclear palsy, which have different misfolded tau structures. The RING-Bait therapy was able to prevent tau aggregation induced by proteins from both Alzheimer’s and progressive supranuclear palsy patient brains.
Prof Leo James, co-leader of the studies, from the MRC LMB, said: “Neurodegenerative diseases can have tau proteins that misfold in many different ways, raising the possibility of needing a different treatment for every disease. A useful aspect of RING-Bait is because it is attached to a tau protein, it’s a universal Trojan horse that should be incorporated into different types of tau aggregates exactly like the cell’s own misfolding tau protein.”
Mice walk better after therapy
For the treatment to work in an animal, it needs to not only get into the brain, but also get inside the cells within the brain. To do this, the researchers used a harmless virus called an adeno-associated virus (AAV), to deliver the DNA instructions to make the custom proteins inside cells.
Aged mice with tau protein aggregates were injected with a single dose of the gene therapy vector containing either the treatment, or a placebo. Within a few weeks, there was a significant reduction in the amount of aggregated tau in the brain cells of the treated animals.
Importantly, in the mice given the RING-Bait treatment, the progression of their neurodegeneration symptoms slowed and they showed significantly better motor function, as assessed by an AI programme that scored how well they ran.
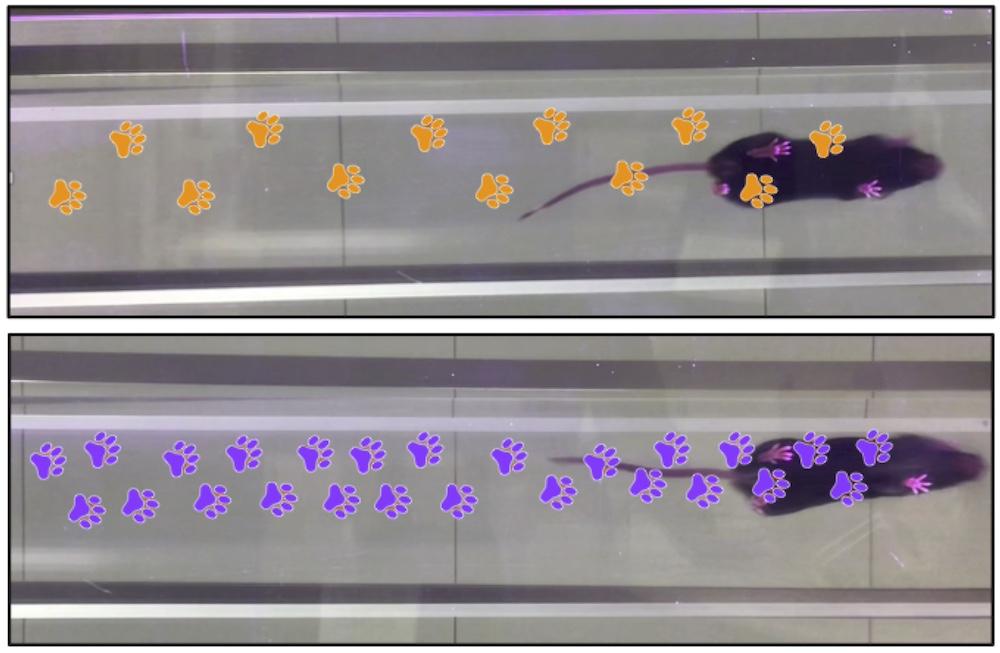
The gait of mice improved after treatment with RING-Bait (top, orange). Mice given the placebo retained poor gait (bottom, purple). Credit: Lauren Miller (MRC LMB)
Dr Lauren Miller, who worked across both the UK DRI and MRC LMB, said:
“It was unknown whether specifically removing tau aggregates inside the cell would be enough to halt the progression of disease. It is encouraging that a RING-Bait approach reduces disease severity in our model systems, as this suggests that the selective removal of tau aggregates is a valid therapeutic approach. Further work will be needed to demonstrate this beneficial effect is found across multiple models of human disease.”
Dr Guido Papa, from MRC LMB, said:
“The beauty of RING-Bait lies in its broad adaptability and the potential to tackle other conditions characterised by the accumulation of pathological protein clusters. Other neurodegenerative diseases are caused by aggregates formed by other proteins, such as TDP43 in motor neuron disease and alpha-synuclein in Parkinson’s disease. It is hoped that RING-Bait will allow the development of future therapies that directly target the aggregation process in these diseases.”
The scientists caution that these therapies still require a lot of development before they can be tested in humans, particularly developing an AAV vector that can safely and effectively deliver RING-nanobody or RING-bait therapies to cells throughout the human brain.
Dr Jonathan Benn, from the UK DRI at Cambridge, said:
“It’s important to stress that although we have shown it works in a mouse model, this is a long way from a therapeutic that could be used in humans. It would need to be determined that it is safe to use TRIM21-based therapies in the human brain and that the treatments are effective in both removing aggregates and improving the course of disease.
“Some AAV vectors are already approved for use in humans – for instance in degenerative eye diseases and genetic diseases like spinal muscular atrophy. However, getting enough AAV into the adult brain remains a significant challenge - the human brain is about 1,000 times bigger than a mouse brain. But this is a rapidly moving field and there are cutting edge gene delivery methods that we hope will allow our therapies to be delivered at scale in the future.”
The researchers say this promising approach could also be applied in future to other brain disorders driven by protein aggregation inside cells, such as motor neuron disease, Huntington’s disease and Parkinson’s disease.
These studies were primarily funded by Wellcome, MRC, UK DRI, and The Lister Institute of Preventative Medicine.
Source: MRC LMB
References:
Co-opting templated aggregation to degrade pathogenic tau assemblies and improve motor function. Miller, L.V.C., Papa, G., Vaysburd, M., Cheng, S., Sweeney, P.W. Smith, A., Franco, C., Katsinelos, T., Huang, M., Sanford, S.A.I., Benn, J., Farnsworth, J., Higginson, K., Joyner, H., McEwan, W.A., and James, L.C. Cell
Aggregate-selective removal of pathological tau by clustering-activated degraders. Benn, J., Cheng, S., Keeling, S., Smith, A.E., Vaysburd, M.J., Böken, D., Miller, L.V.C., Katsinelos, T., Franco, C., Dupré., Danis, C., Landrieu, I., Buée, L., Klenerman, D., James, L.C., and McEwan, W.A. Science
Banner image: Sophie Sanford