A unique protein normally involved in the body’s immune response to viruses could hold the key to safer immunotherapy treatment for Alzheimer’s, says Prof Will McEwan, an immunologist aiming to selectively target toxic proteins in neurodegenerative disease.
This article is part of our ‘Develop’ series, showcasing the UK DRI's mission to discover the causes of neurodegeneration, develop possible treatments and deliver solutions for healthy ageing.
A childhood interest in the natural world inspired Prof Will McEwan to pursue a career in science. By the time he finished university, his curiosity had grown (or shrunk) even beyond the microscopic, to the world of viruses. Today Will leads a lab at the UK DRI at Cambridge, harnessing his background in viruses and the immune system in the pursuit of treatments for dementia.
Working with brilliant people, who endlessly surprise me in their capacity to contribute, help each other, generates this incredibly cohesive and good natured sense of purpose, which you see throughout the UK DRI. I feel very lucky to be in this environment.
Group Leader
But how does an immunologist end up working to find treatments for neurodegenerative diseases? The answer lies in a key protein known as Tau. Tau is found to clump together and form toxic aggregates in the brains of people living with Alzheimer’s and other ‘Tauopathies’. It may be the case that the immune system holds the key for dealing with such toxic aggregates.
“In the 2000s there were a lot of papers showing that antibodies targeting tau reduced the amount of aggregates in mouse models, but it was unclear exactly how. Tau largely exists inside cells, while antibodies are normally outside cells, so the question was: ‘they are meeting, but where?’" Will recalls.
Will undertook a postdoc at the MRC Laboratory of Molecular Biology in Cambridge, and it was there that he co-discovered and characterised a particular and unusual receptor for antibodies, TRIM21.
“TRIM21 is inside our cells, and its function is to bind to antibodies. Most of the time, antibodies are patrolling for viruses to latch onto outside cells. Occasionally they bind to a virus, and when that virus infects a cell, the antibodies come inside the cell attached to the virus. TRIM21can then bind to the antibody and break down the virus. We started thinking about whether we could use this response against other things, such as tau.” he explains.
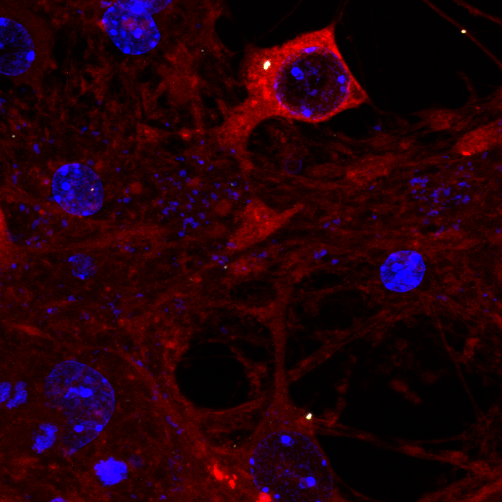
Tau assembly being attacked by TRIM21 inside a neuron
Since then, Will has been combining the fields of immunotherapy and neurodegeneration, with his sights set on taking his findings forward into the clinic.
A ‘tuneable’ antibody receptor
Although antibody therapies are a widespread and successful tool in modern medicine, one of the main risks they pose is in generating excessive inflammation. This is particularly problematic in the brain, where in the most severe instances it can trigger brain swelling and bleeding. These are the main side effects of the new Alzheimer’s treatments such as lecanemab and donanemab.
“So, if you're trying to use antibodies to treat something like tau pathology, you provoke an immune response in the brain. Antibody therapy can be quite inflammatory, unfortunately.” Will explains.
This is where TRIM21 comes into play: rather than binding to external antibody receptors and generating inflammation, the McEwan Lab have shown that antibodies selectively binding to TRIM21 can avoid these unwanted inflammatory effects. Once bound, TRIM21 ‘tags’ tau for destruction via the cell’s waste disposal pathways.
“TRIM21’s function is to generate a tag that the cell recognises for degradation, and it does so very effectively,” he says.
This enzyme is inert most of the time, and only kicks into action by triggering a defensive response when it detects something that needs to be degraded. This provides an opportunity for managing the process.
“That means TRIM21 is tuneable to us,” Will explains.
Delivering safer treatments
The McEwan Lab has several projects underway. Two recent studies published in Science and Cell demonstrate how TRIM21 can be harnessed to break down aggregated tau in mouse models. In these new publications, the scientists used TRIM21 to create two experimental therapies and show that they significantly reduced the amount of toxic tau in the brains of the mice. Using the new-found tuneable nature of TRIM21, they showed that soluble, healthy tau could be spared, leaving it able to continue its job.
Will and his team are set on taking these findings forward to further understand the biology of how aggregates can be degraded, and how these findings could be advanced towards new treatments. After all, as he says, his work is driven by two determining factors: on the one hand, the collaborative and enriching team environment; on the other, the desire to come up with new insights into unmet clinical problems.
Image credits: Lauren Miller (MRC Laboratory of Molecular Biology), Annabel Smith (McEwan lab, UK DRI).