Biography
Dr. Marc Aurel Busche is a psychiatrist and neuroscientist at the UK Dementia Research Institute at UCL.
His major focus is to understand the cellular and circuit mechanisms that underlie the initiation and progression of Alzheimer’s Disease (AD) and related disorders so that, through this understanding, more effective therapies can be developed. He also provides patient care as an honorary consultant psychiatrist in the cognitive disorders clinic at the Queen Square National Hospital for Neurology and Neurosurgery.
Dr. Busche attended medical school at the Ludwig Maximilians University of Munich, and completed his clinical training in psychiatry and psychotherapy at the University Hospital of the Technical University of Munich. He performed his graduate work with Arthur Konnerth at the Technical University of Munich, carried out research as a Tandem Group Leader at the Munich Cluster for Systems Neurology and also performed an EMBO Fellowship at Massachusetts General Hospital and Harvard Medical School with Bradley Hyman.
He is the recipient of several awards and fellowships including a UKRI Future Leaders Fellowship.
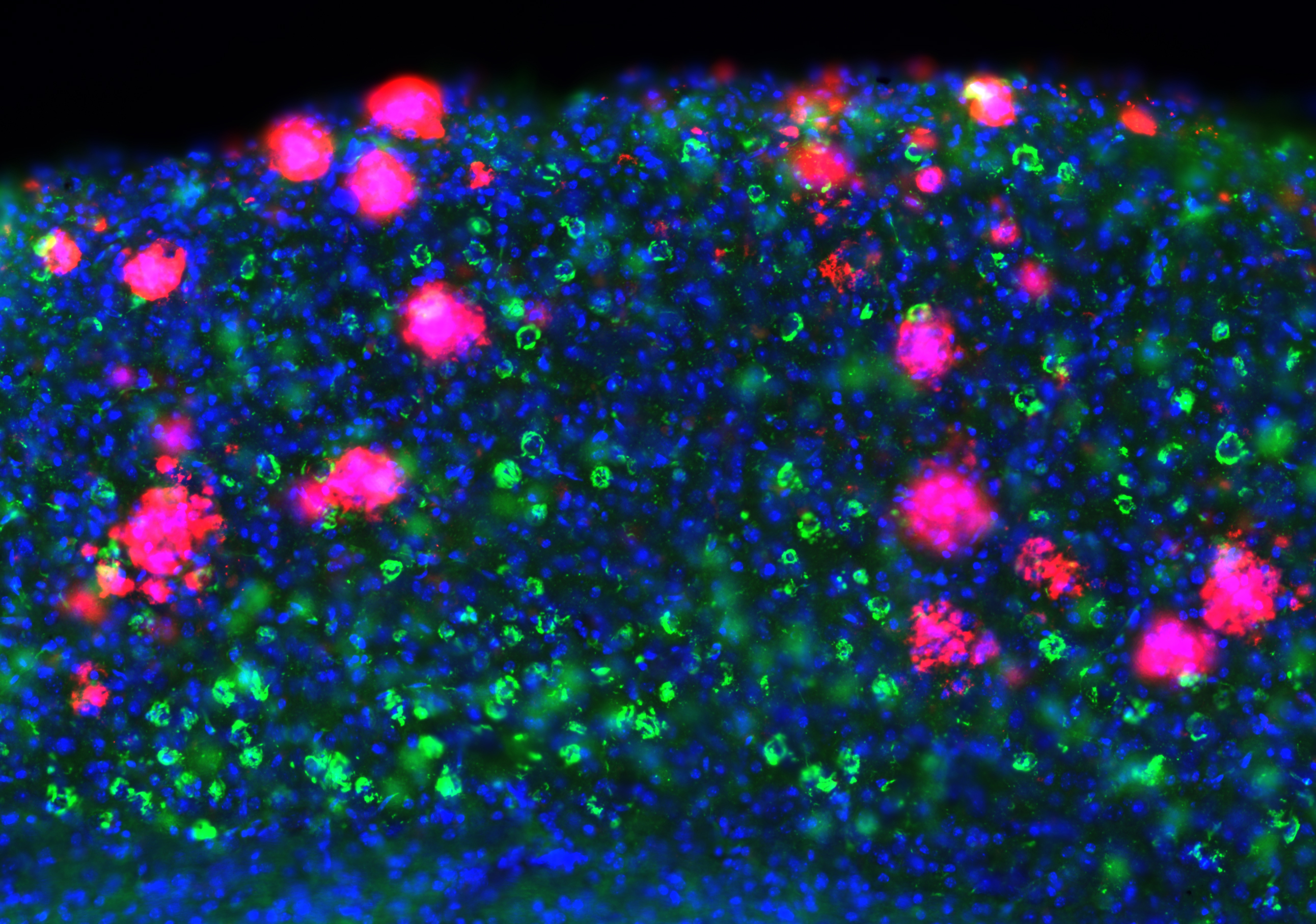
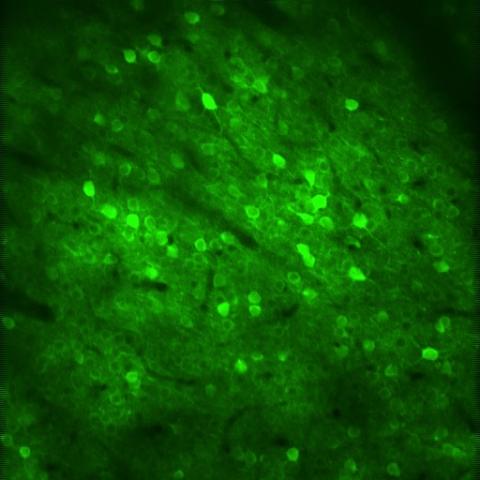
Busche Lab
Explore the work of the Busche Lab focused on understanding and repairing pathological neural circuits in Alzheimer's disease.