Info
Protecting neurons from harm as we age
Over the course of our lifetime, the DNA inside our cells is under constant attack – either from dangerous metabolic by-products such as reactive oxygen species, or external factors. To counter this threat, our bodies have evolved elaborate tools that can spot and repair damaged DNA. However, when these systems go wrong, a build-up of genetic faults can occur and disastrous consequences follow, such as neuronal loss.
Most neurons – the building blocks of our central nervous system – are created early on in life and are never replaced. Our body needs to protect these cells from harm for the 80 or more years that we are alive – otherwise, this can lead to devastating conditions such as Huntington's, Alzheimer’s or Parkinson’s disease. Therefore, scientists are exploring how DNA damage and/or erroneous repair contributes to the loss of function and death of neurons, and the processes that help safeguard these brain cells as we age.
The Balmus Lab is developing a sophisticated new experimental system to identify genetic and environmental factors involved in DNA damage, neurodegeneration and ageing in neurons. He hopes this will uncover key biological targets that will lead to the development of effective new treatments that can help protect diseased neurons from degeneration.
Latest news


Prof Gabriel Balmus
Prof Gabriel Balmus is a Group Leader at the UK DRI at Cambridge. Find out more about his career and expertise on his profile page.

Research summary
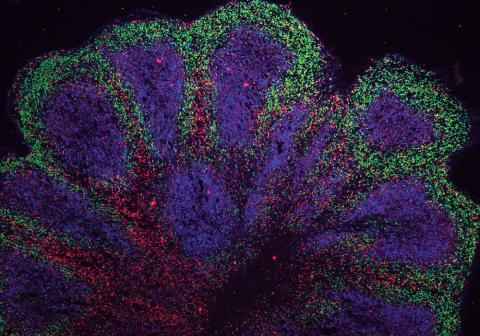
A human brain organoid used to model Huntington's disease. Credit: Balmus Lab.
Identifying neuroprotective mechanisms against genomic instability accrual in ageing and neurodegeneration
Genomic instability (GIN) is an important feature of neurodegeneration and a hallmark of neuronal ageing; if therapeutically averted, this could lead to prevention, delay or reversal of GIN-related neurodegenerative disease. Most mature neurons are end-state (non-replicative) cells that need to live for 80+ years and over this time require the ability to deal with exogenous and endogenous factors that create DNA lesions (DNA damage). In mature neurons, these lesions are counteracted by the DNA damage response (DDR) via mechanisms that are as yet unclear. It is proposed that failure to impede GIN formation and/or execute accurate DNA repair will cause expansion of nucleotide repeats, cell cycle re-entry, loss of synapses, chronic inflammation, premature senescence, aggregation of proteins or cell death - processes that would lead to the initiation of diverse neuropathology. Hence, accumulation of GIN throughout life represents one of the hallmarks of numerous neurodegenerative diseases including Huntington’s Disease (HD), Amyotrophic Lateral Sclerosis (ALS), Parkinson’s Disease (PD) and Alzheimer’s Disease (AD).
Although GIN is likely to be a central player in neurodegeneration, either in specific pathologies or ageing, to date there is no comprehensive understanding of the cause-effect relationship nor are there any approved therapies to combat neurodegeneration via maintenance of genomic stability. Currently, a major obstacle is a lack of cellular models that can be used to study relevant neurodegenerative-related cellular states in human mature neurons. In terms of the GIN connection, this is mainly due to the fact that most of the DDR-related research has been done in replicative transformed cell lines, usually of mesenchymal/cancer origin or GIN unstable, non-isogenic induced pluripotent cell lines (iPS). However, studying the DDR in any cell type other than genomically stable human/mouse embryonic stem cells that can be used for the derivation of mature neurons can produce dangerous inaccuracies.
Main objectives and research goals:
The overall aim of this research programme is to identify genetic factors that can re-balance GIN-related neurodegeneration in mature neurons and understand how these molecular processes contribute to neurodegenerative disease such as HD, ALS, PD or AD, as well as ageing. The specific aims are:
- To study GIN-related neurodegeneration, develop relevant experimental disease models in both mouse and human isogenic ESCs that can be rapidly differentiated into neurons (iN) and/or organoids.
- Using CRISPR-Cas9 and/or chemical mutagenesis to screen for factors that can rescue neurodegeneration upon increased endogenous (i.e. reactive oxygen species) damage in WT and selected disease backgrounds such as HD, ALS or PD.
- Once modifiers are identified in screens or based on hypothesis driven research, understand the molecular mechanisms basis for re-balancing disease towards designing new drugs or re-purposing already available therapies.
- Validate the relevant diagnostic and therapeutic targets and/or compounds in patient iPS and organoids as well as subsequent in vivo studies.
Vacancies
Key publications
Lab members
- Dr Matthew Ellis (Postdoctoral Researcher)
- Dr Rizwan Ansari (Postdoctoral Researcher)
- Dr Mihai Miclaus (Senior Bioinformatician)
- Dr Laura Morcom (Postdoctoral Researcher)
- Dr Ioana Ardelean (Postdoctoral Researcher)
- Dr Dragos Leordean (Postdoctoral Researcher)
- Dr Andrei-Stefan Lia (Postdoctoral Researcher)
- Nadia Karimpour (Lab Manager/Research Assistant)
- Michael Woods (Vivo Research Manager)
- Osama Bin Faisel (PhD Student)
- Jose Vicente(Zé) (PhD Student)
- James Woodward (PhD Student)
- T.T. Yang(Denny) (PhD Student)
- Catalin Coltau (PhD Student)
- Irina-Maria Ungureanu (PhD Student)
Collaborators







Lab funders
Thank you to all those who support the Balmus Lab!