Developing treatments against dementia in people with Down syndrome
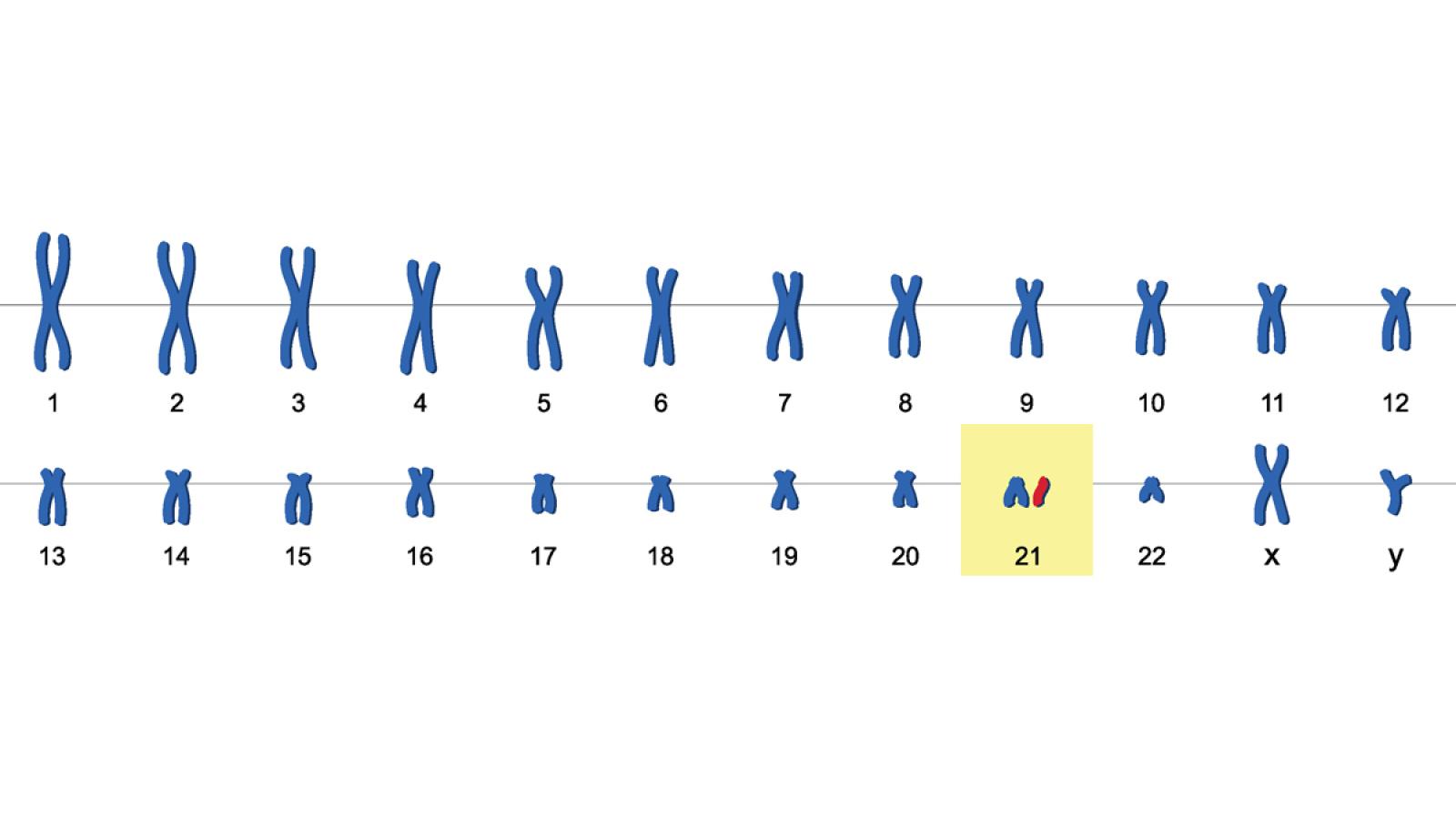
There are an estimated 6 million people living with Down syndrome worldwide. Down syndrome is a genetic condition caused by having an extra copy of chromosome 21. People who have Down syndrome are at greatly increased risk of developing early-onset dementia caused by Alzheimer’s disease, and in the UK, Alzheimer’s disease-dementia is the leading cause of death for adults who have Down syndrome. Importantly, newly developed Alzheimer’s disease treatments are currently not recommended for use in people who have Down syndrome, because of differences in disease caused by the extra copy of chromosome 21.
The Wiseman Lab aims to understand how the extra copy of chromosome 21 affects the development of Alzheimer’s disease in people who have Down syndrome. Their work particularly focuses on how the extra copy of chromosome 21 affects the development and response to amyloid-β accumulation within the brain. This work aims to inform the safe use of new Alzheimer’s disease therapeutics developed for the general population, in an important group of individuals at high risk of early disease and to identify new therapeutic targets by the study of the most commonly occurring genetic cause of Alzheimer’s disease.
Latest news

Dr Frances Wiseman
Dr Frances Wiseman is a Group Leader at the UK DRI at UCL, and Programme lead for Animal Models for the Institute. Find out more about her career and expertise on her profile page.

Research summary
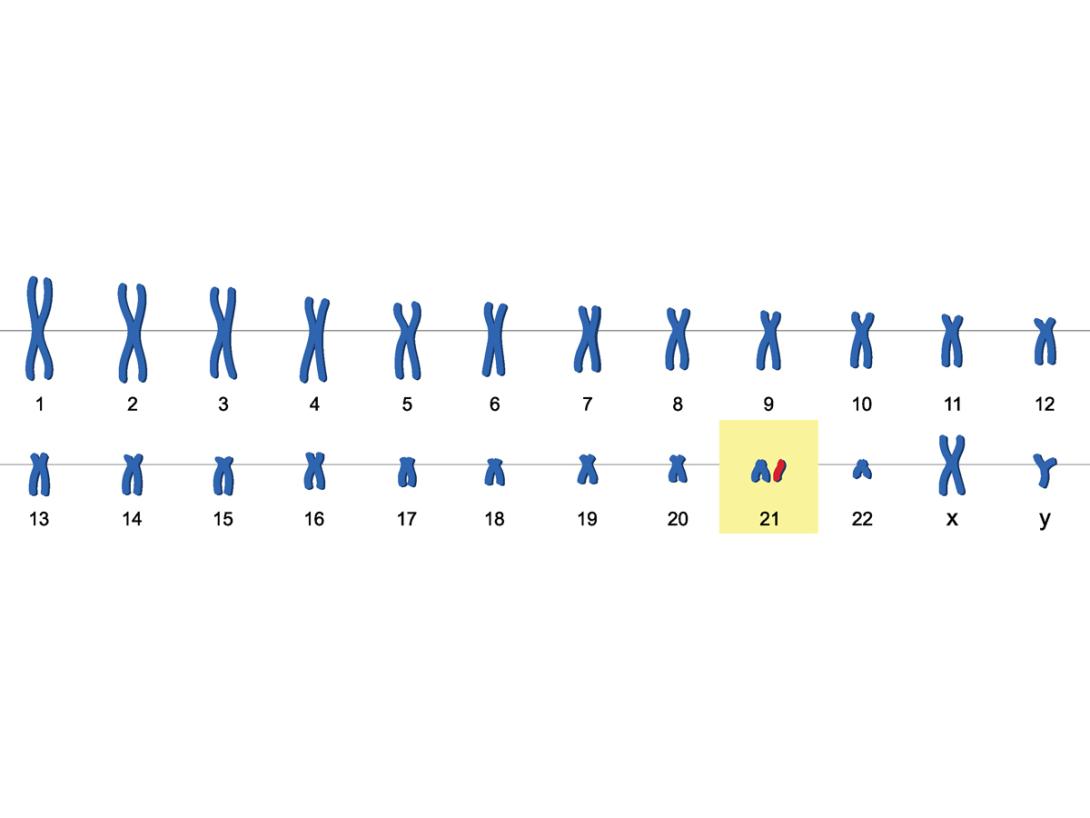
Down syndrome is caused by an additional copy of chromosome 21. The gene for the amyloid precursor protein (APP) is found on this chromosome, and linked to the high risk of dementia associated with the condition. Credit: Shutterstock/Ody_Stocker
Mechanisms of Alzheimer's disease development in Down syndrome
Down syndrome or trisomy of chromosome 21 is the most commonly occurring genetic cause of early-onset dementia (Down syndrome Alzheimer’s disease DSAD). Individuals with the condition experience accelerated amyloid-β accumulation within their brains resulting from the additional copy of APP, which leads to early-onset dementia with a mean age of clinical dementia diagnosis of between 53-55 years. In addition to this, Down syndrome also alters the development and function of many major organs and molecular pathways. How these changes affect the progression of AD and the safety and efficacy of AD therapies is poorly understood, and this has significant clinical implications for this large group (~6 million worldwide) of individuals who are at a highly elevated risk of developing dementia early in life.
The Wiseman Lab use an integrated research programme combining mouse and iPSC derived cellular models and studies of human post-mortem tissue to understand how trisomy of chromosome 21 affects AD-relevant processes and the progression of disease. This has included a series of preclinical studies demonstrating that an additional copy of chromosome 21 genes, other than APP, affects the development of amyloid-β pathology (Wiseman et al 2018, Tosh et al 2021, Mumford et al 2022) and that key differences occur in brains of people who had DSAD compared with age-matched cases of AD in the general population (Wu et al 2023). Importantly, the development and function of the immune system is significantly affected by trisomy of chromosome 21, but how this altered biology interacts with AD development and progression is poorly understood. In particular, recent research has highlighted that trisomy 21 leads to a hyperactivation of the interferon response, because of the location of four interferon receptor genes on chromosome 21. Dr. Wiseman's team are determining how this altered biology affects the formation of and response to amyloid-β pathology, with a focus on neuropsychiatric changes which occur in the prodromal phase of DSAD.
Main objectives
- To determine if trisomy of Hsa21 is sufficient to alter interferon signalling in response to amyloid-β accumulation.
- To determine if trisomy of Hsa21-associated changes to interferon signalling result in an alteration to prodromal features of DSAD with a focus on neuropsychiatric changes.
- To determine which brain cell-types are critical for trisomy Hsa21-associated changes to interferon signalling and clinically relevant downstream alterations.
Watch a recent full lecture given by Frances here
Key publications
Lab members
- Dr Sevda Boyanova (Postdoctoral Researcher)
- Dr Cliona Farrell (Postdoctoral Researcher)
- Paige Mumford (Research Assistant/PhD Student)
- Millie Beament (Masters Student - joint with Prof Nick Fox)
- Fukko Kammuri (PhD Student)
- Hannah Mager (PhD Student)
- Benedetta Dentone (Student)
- Daizi Davies (Student)
- Aditi Bisen (Student)
Collaborators













Lab funders
Thank you to all those who support the Wiseman Lab!