The use of animals remains a vital feature of modern research, allowing scientists to model and interrogate key aspects of disease. There are however limitations when mapping animal biology and behaviour to humans, many of which are blamed for poor translation from pre-clinical research to the patient. A new company, Cambridge Phenotyping Ltd., aims to tackle several of the common issues plaguing rodent behavioural work, with their novel automated and AI-assisted ‘smart-Kage’ technology.
“So just imagine that you have your mouse, which has received no training. You put it in the cage, close it up and walk away, that's it,” explains lead developer of the technology and CEO of the new company, Dr Julija Krupic. “And the system does all the testing for you, 24/7, with the data put out in near real time so you can run all the analysis that same day.”
If you have spent even a day performing a rodent behavioural task, the process Dr Krupic describes, will no doubt be music to your ears. Assessing the change to an animal’s behaviour should be one of the best methods for tracking the onset, progression and perhaps treatment of a disease, and crucially relating that to a human condition. This is especially true for a long, progressive disease like Alzheimer’s but, as Dr Krupic discovered, the protocols currently established often come up short.
“It became very clear to us that we didn’t have good criteria to define the onset, middle and end of disease in these Alzheimer’s models. We were trying our best but still picking arbitrary timepoints. What we really needed was very stable, continuous testing of our animals.”
Setting out to develop a solution, Dr Krupic, a physics graduate, teamed up with Dr Marius Bauza, a Senior Research Fellow at the Sainsbury Wellcome Centre. Funded by a collaborative grant from UK DRI Director Prof Bart De Strooper, what emerged was a 50 x 50 x 50cm cube, dubbed the ‘smart-Kage’, as Dr Krupic discusses.
“It’s a very similar environment to other cages from the mouse’s perspective. It has a nest, water bottle, scattered food pellets and environmental enrichment like a running wheel. The main difference is a large, fixed camera built into the lid which records footage of the mouse, later analysed by the artificial intelligence system.
There are several advantages to the set-up. Firstly, animal work is labour intensive, especially if you have to spend weeks training the rodent to perform new tasks. Here you can put the mouse in and basically begin behavioural assessment immediately. The handsfree approach also removes factors that interfere with results such as stress brought about by unnecessary handling and food deprivation protocols sometimes required in training. In addition to continuous monitoring, which we’ve tested in a proof-of-concept for 12 months, we have in-cage infrared lights set up to control important light-dark cycles as rodents are nocturnal. For all of these reasons and more, we hope the system is a much more efficient, smart method for long-term behavioural assessment.”
Standard smart-Kage dimensions will be evolved to fit different animal facilities
Applying the technology
Dr Krupic’s research background lies in the fundamental neuroscience underlying navigation and spatial memory, studying the special arrangement of place and grid cells in the brain – follow-up work to that which earned her PhD supervisor Prof John O’Keefe a Nobel Prize. After some time spent in the States, Dr Krupic now leads a group at the University of Cambridge as one of Wellcome’s prestigious Sir Henry Dale Fellows, undertaking research with a more translational edge.
“At UCL I carried out two-photon imaging of the brain while the mice were in virtual environments. The areas of the brain we're interested in for spatial memory and navigation, the entorhinal cortex and hippocampus, are actually some of the first to be afflicted in Alzheimer’s disease. I’m looking to find out what might be going wrong in the earliest stages, and the behavioural mouse work is important for this.”
Putting the smart-Kage technology through its paces, the team have now tested three common behavioural tests which assess cognitive function, particularly involving the brain regions of the entorhinal cortex and hippocampus: T-maze, novel-object-recognition, and object-in-place recognition. The 24/7 tracking of movement also allows the researchers to monitor periods where the mice are motionless and assumed to be sleeping, matching well with light-dark cycles of the system.
Dr Krupic tests have also extended to disease paradigms including a mouse model with a lesioned hippocampus, showing the technology works as well as or better than previous methods. Additionally, new collaborations with UK DRI Centre Director Prof Karen Duff, UK DRI Group Leader Dr Frances Wiseman and Dr Sara Wells from MRC Harwell, have allowed the team to explore models of amyloid and tau pathology characteristic of Alzheimer’s. Dr Wiseman, who also leads the Animal Models Programme at the UK DRI, discusses the issues with current behavioural methods and the potential of the new smart-Kage technology.
“The brain’s fundamental function is to control behaviour and studying how these processes are altered by dementia-causing diseases is central to the work of the UK DRI. Conventional behavioural testing of rodents is extremely labour intensive, relies on drivers of response such as fear or hungry that may confound studies and often has poor reproducibility; limiting the translational value of this research. Novel paradigms like smart-Kage, that enable the collection and interpretation of spontaneous behaviours of rodents, offer enormous potential for future dementia research”.
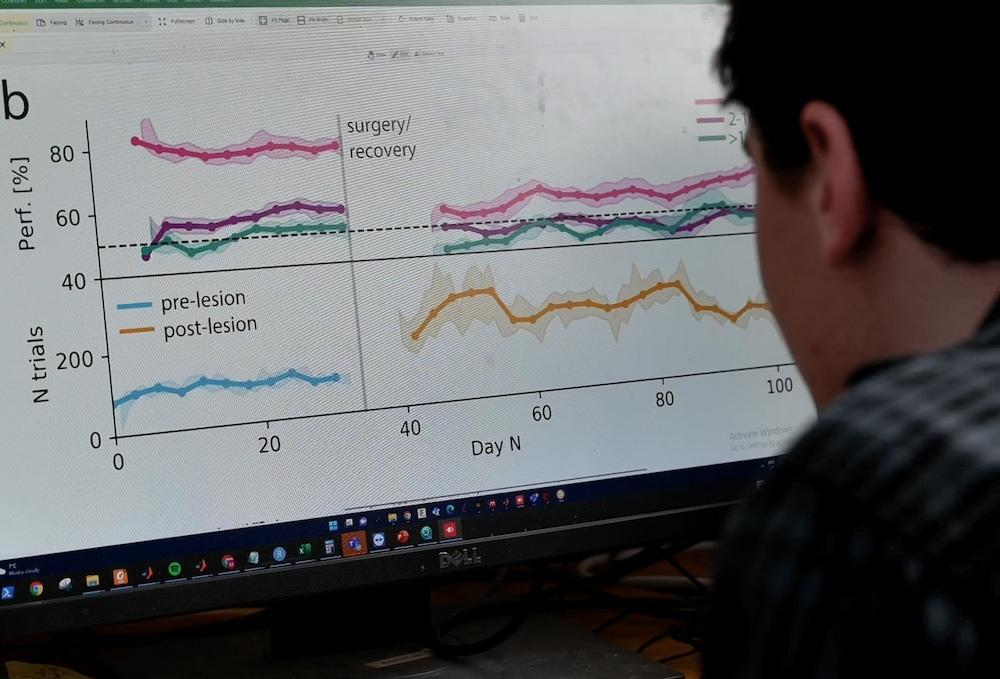
In proof-of-concept studies the smart-Kage performs as well as or better than conventional behavioural testing methods
Scaling up
While setting out to develop the system, Dr Krupic explains this wouldn’t have been possible without a drop in prices of key components and wider access delivered through technological breakthroughs, often from somewhat unexpected sources.
“There has been a huge advancement in super-fast, powerful graphics cards for gaming. I even received some funding from the Nvidia, a giant in this area, who were interested in the application for basic research. Arduino, an electronic microcontrollers, and Raspberry Pi, a small affordable computer used for things like cameras in car, have also made all of this possible.”
The new start-up Cambridge Phenotyping Ltd. is now taking orders for the smart-Kage after finalising a license agreement this month. Dr Krupic admits there is still room for more development, but also much potential utility for the technology.
“Our big ambition is to create a global database which everyone feeds data into. This would be freely accessible to anybody interested in animal disease models, and a step up from classic characterisation with more sophisticated side-by-side comparison using the smart-Kage.
We are still working on adapting the cages so that they can be practically rolled out in animal facilities across the world, for instance, by modifying the system to fit in different kinds of racking and be run through washing machines of differing sizes. I believe this technology not only produces better results but saves precious resources and researcher time.
I’m excited to see how far we can push it with the variety of tests. There is also the potential to carry out direct in vivo neural recording while simultaneously monitoring the behaviour. We have even been in touch with researchers from different disease fields.”
With the ultimate aim of improving translatability between basic research and the clinic, Dr Krupic is additionally driving a project assessing behaviour in humans directly, using a smart phone app.
“We ask people to play this very short game which takes just six seconds per interaction, around 15 times a day. It relies on your ability to remember previous choices you’ve made in the game. We’ve only performed it with a small cohort of people, but our aspiration is to monitor people’s cognitive health over time. Like a lot of the tests in mice, we are assessing the function of the hippocampus and something called spatial working memory. The real strength is in developing and running tests that are directly comparable between animal and human research.”
Read more about Dr Julija Krupic on her University of Cambridge profile and lab website. Further information on the smart-Kage technology and access is located on the Cambridge Phenotyping Ltd. website. Find out more about the UK DRI’s translational strategy and activities on our dedicated ‘translation and innovation’ webpage, and sign up to our quarterly newsletter for the latest updates in this area.
Article published: 20 April 2022
Images courtesy of Cambridge Phenotyping Ltd.
