Biography
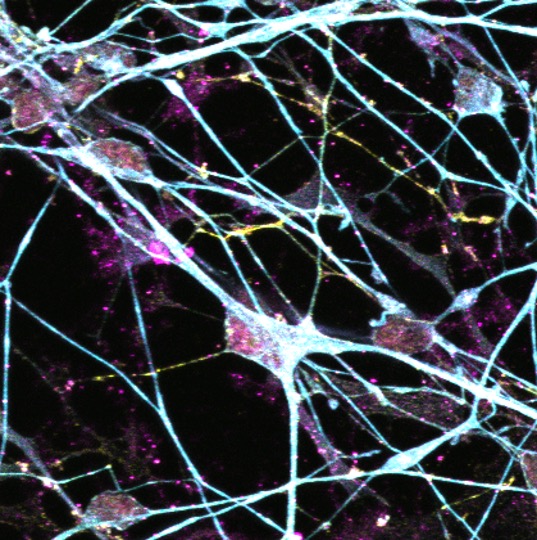
Prof Tara Spires-Jones’ FMedSci research focuses on the mechanisms and reversibility of neurodegeneration in Alzheimer’s disease, other degenerative brain diseases, and ageing. Her work has shown that soluble forms of pathological amyloid beta and tau contribute to synapse and neurodegeneration and that pathological forms of tau spread through the brain via synapses.
In addition to research, Prof Spires-Jones is passionate about communicating scientific findings to the public and policy makers; increasing the rigour and reproducibility in translational neuroscience; promoting inclusivity and diversity in science; and supporting career development of neuroscientists. She is President of the British Neuroscience Association (2023-2025), Director of the University of Edinburgh Centre for Discovery Brain Sciences, and was elected to the UK Academy of Medical Sciences in 2024.
Spires-Jones Lab
Explore the work of the Spires-Jones Lab focused on deciphering why synapses and neurons degenerate and whether boosting resilience of synapses can protect the brain.