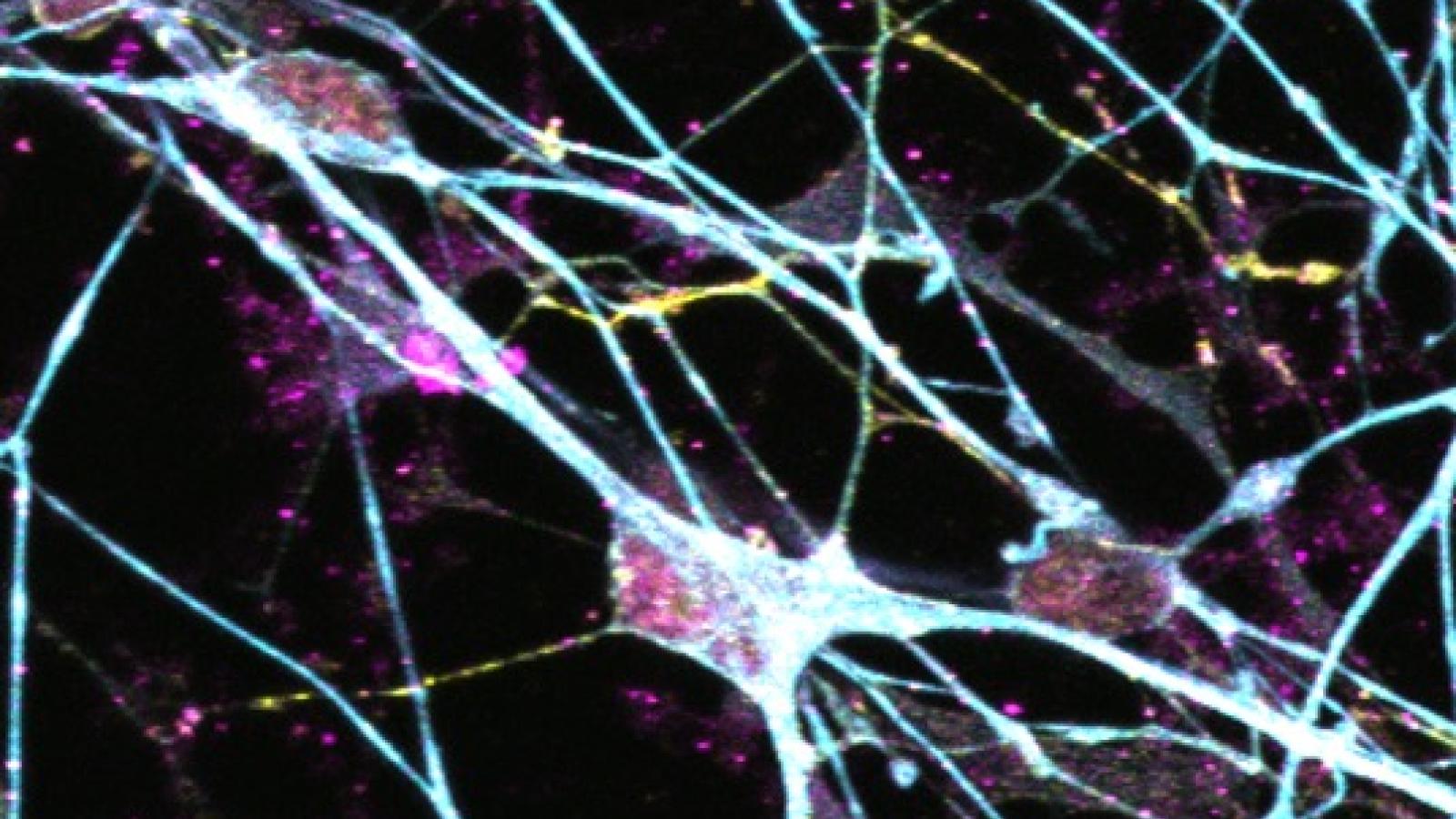
Key details
Losing brain connections in dementia
Memory is made possible by the ability of synapses, the connections between neurons in the brain, to change in response to environmental inputs. In dementia, memory declines because synapses and neurons become dysfunctional and die. In fact, loss of synapses is a strong predictor of dementia symptoms in people living with Alzheimer's disease.
The goal of the Spires-Jones Lab is to understand why synapses and neurons degenerate and whether boosting resilience of synapses can protect the brain. In the long term, the team aims to use what we discover to develop effective strategies to prevent and treat Alzheimer's and related brain diseases.
Latest news


Prof Tara L Spires-Jones
Prof Tara L Spires-Jones FMedSci is a founding Group Leader at the UK DRI at Edinburgh. Find out more about her career and expertise on her profile page.

Research summary
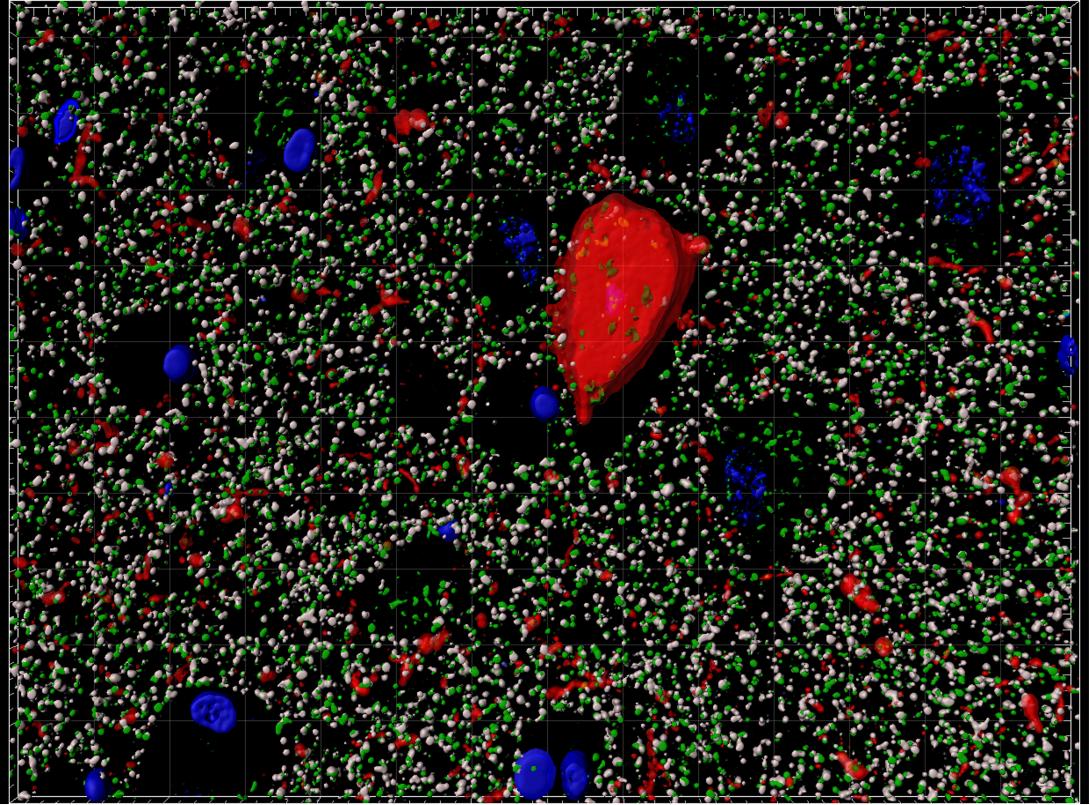
Human neurofibrillary tangle, nuclei, presynapses, postsynapses imaged with array tomography. Credit: Caitlin Davies
Non-cell autonomous mechanisms of synapse pathology
Prof Spires-Jones' research focuses on the mechanisms and reversibility of neurodegeneration in Alzheimer’s disease, other degenerative brain diseases, and ageing. Working with a vibrant group of researchers, Tara is trying to understand mechanisms of synapse degeneration and the trans-synaptic spread of tau pathology and how astrocytes and microglia may be involved in these processes.
Using pioneering imaging technques, her work has shown that soluble forms of the pathological proteins amyloid beta, tau, and alpha-synuclein accumulate in synapses in post-mortem human brain samples from people who died with Alzheimer’s disease, other tauopathies, and synucleinopathies. Further, her team provided evidence that tau pathology spreads through the brain via synapses.
They have also discovered that in human brain, both astrocytes and microglia engulf synapses with more astrocyte engulfment during ageing and Alzheimer’s disease. Current work aims to understand whether glial engulfment of synapses is mediated by synaptic activity or responses to pathological protein accumulation. The team is also looking for molecular interactions mediating trans-synaptic tau spread to find therapeutic targets to stop the progression of tau pathology through the brain and the associated neuron loss.
Key publications
Vacancies
Lab members
- Jane Tulloch (Lab and Animal Manager)
- Declan King (Postdoctoral Researcher)
- Francesco Gobbo (Postdoctoral Researcher)
- Sowmya Sekizar (Postdoctoral Researcher)
- Manuela Marescotti (Scientific Editor)
- Elizabeth Simzer (PhD Student)
- Kris Holt (PhD Student)
- Edmond Mouofo (PhD Student)
- Rob McGeachan (PhD Student, jointly with Dr Claire Durrant)
- Laoise Casserly (PhD Student, jointly with Prof Giles Hardingham)
- Lauren Young (Student)
Collaborators











Lab funders
Thank you to all those who support the Spires-Jones Lab!