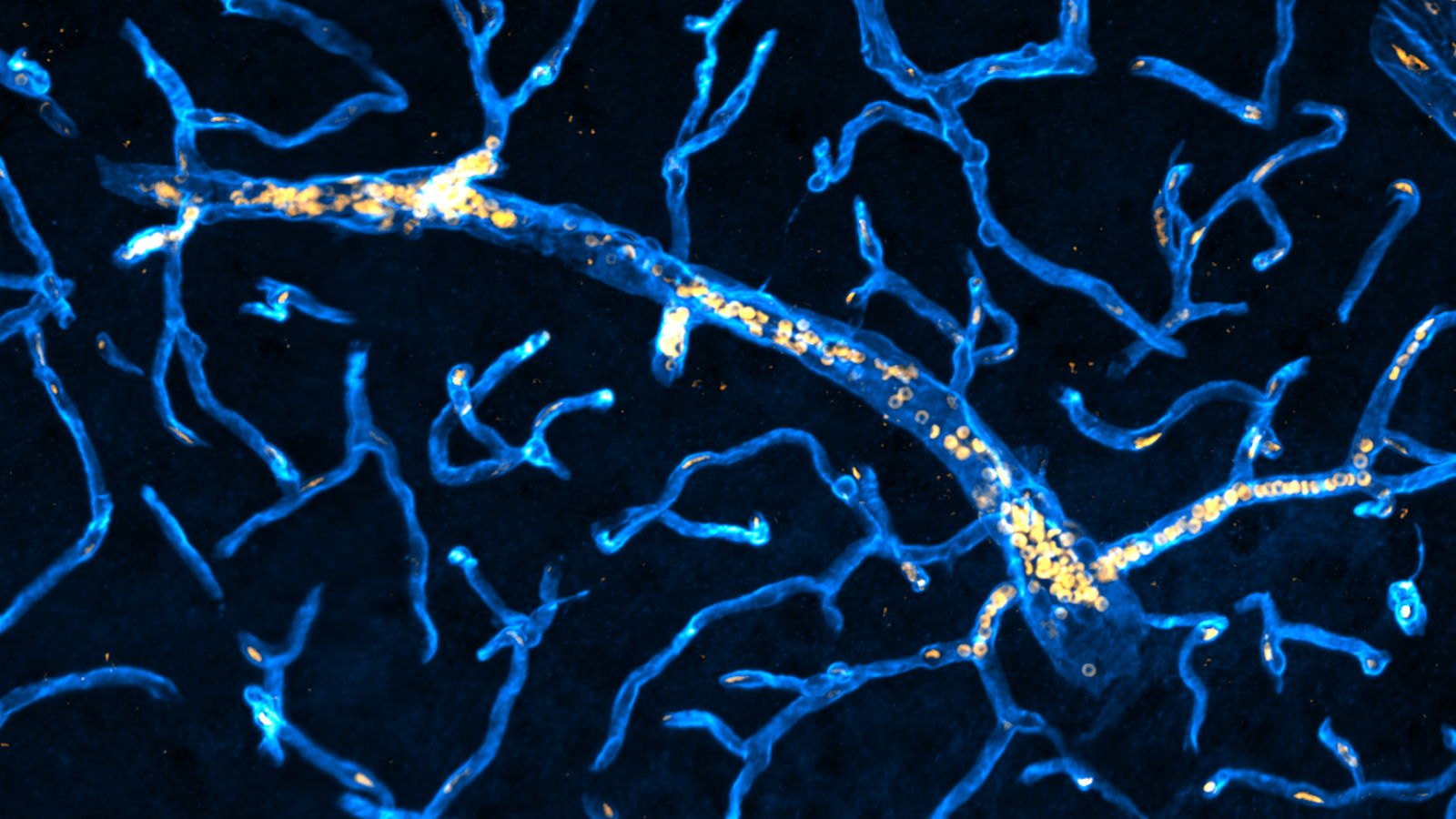
Key details
Understanding how, when, and where pericytes lining the blood-brain barrier become dysfunctional
Our brain is an energy-hungry organ surrounded by a rich network of blood vessels supplying the oxygen and nutrients required to function. It is essential that the microenvironment in the brain is finely controlled, and this is achieved through the specialist blood-brain barrier (BBB) structure. However, dysfunction of the BBB is recognised as one of the earliest events in the progression of brain disorders that cause dementia, and scientists are working to understand why this occurs.
The Montagne Lab has previously discovered that one type of cell within the BBB, the pericyte, is particularly affected during disease and it aims to fully understand the consequences to the BBB and brain health as a whole. Using a combination of advanced molecular and imaging techniques including MRI, the group seeks to uncover the disease mechanisms at play and identify therapeutic targets for intervention
Latest news


Dr Axel Montagne
Dr Axel Montagne is a Group Leader at the UK DRI at Edinburgh. Find out more about his career and expertise on his profile page.

Research summary

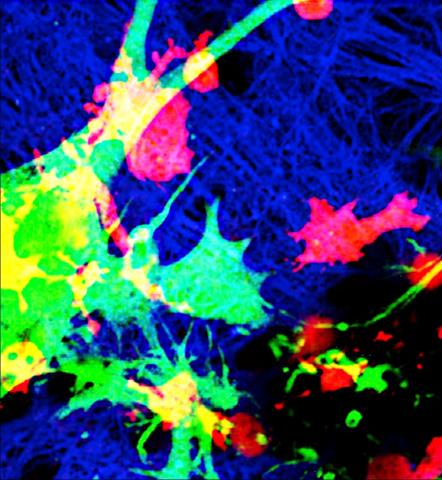
Vascular deserts. Credit: Montagne Lab
Identifying key mechanisms and pathways that lead to impaired BBB function
Many neurological disorders including small vessel disease (SVD) and Alzheimer’s disease (AD) are associated with blood-brain barrier (BBB) dysfunctions. Early degeneration of brain pericytes, activation of endothelial cells, and degradation of the vascular basement membrane (VBM) are thought to be critical factors in the development and progression of common dementias. However, the molecular pathways driving these dysfunctions are largely unknown, with no established therapies.
The overarching aim of the Montagne Lab's programme is to identify key mechanisms and pathways that lead to impaired BBB function which is detrimental to subsequent neuronal and cognitive functions.
The researchers are focusing on the causes of pericyte dysfunction and death, and how pericyte loss destabilises the endothelial cells lining the BBB. In parallel, they are investigating how the VBM, as a central structural and signalling actor, changes in these contexts. Finally, they also investigate the downstream impact of vascular dysfunctions on the brain’s immune response. The group is specifically assessing how brain-resident perivascular microglia can either promote or disrupt BBB integrity. Greater knowledge in these areas may lead to novel therapeutic targets for cerebrovascular protection in chronic neurodegenerative diseases, particularly SVD and AD.
The programme is comprised of three aims:
- Objective A. Assess the impact of modulating both pericyte and endothelial cell functions on VBM composition and function, and how VBM alterations feeds back to further impair BBB integrity. Using innovative genetic mouse models, the Montagne Lab studies how the VBM changes its composition and function with a compromised BBB by combining cutting-edge imaging technologies (i.e., MRI, 2P and confocal microscopy) with analysis of evolving endothelial cell, pericyte, and VBM proteomic signatures.
- Objective B. Determine the role of resident microglia and systemic immune cells in responding to a compromised endothelium-pericyte interplay, and their contribution to BBB integrity. The team assesses how microglia and peripheral immune cells sense and react to acute and chronic BBB dysfunctions using a combination of innovative genetic mouse models, live simultaneous PET-MR and 2P microscopy, and microglia/infiltrated immune cell transcriptomic profiling.
- Objective C. Define the impact of vascular-targeted therapeutic interventions for ameliorating vascular, neuronal, and cognitive functions in the context of SVD and AD. BBB-specific genetic and/or pharmacological modifications aiming to prevent pericyte loss, endothelial activation, and restore BBB functions. The researchers also assess the impact of these vascular-targeted interventions on neuroinflammation, as well as downstream key outcomes measures that are neuronal and cognitive functions. These findings may support further development of this therapeutic strategy for clinical use.
Key publications
Vacancies
Lab members
- Dr Audrey Chagnot (Postdoctoral Researcher)
- Dr Conor McQuaid (Postdoctoral Researcher)
- Dr Ogochukwu J. Uweru (Postdoctoral Researcher)
- Nela Fialova (Research Assistant)
- Solvig Becker (Research Assistant)
- Daniela Jaime-Garcia (Wellcome Trust Translational Neuroscience PhD Student)
- Krystal Laing (Wellcome Trust Translational Neuroscience PhD Student)
- Dorota Stefancova (PhD Student)
- Daniel Reti (PhD Student)
Collaborators




















Lab funders
Thank you to all those who support the Montagne Lab!