Key details
Using advanced forms of MRI and PET brain scanning
Alzheimer’s disease (AD) brain changes begin decades before symptoms. Initially, abnormal proteins are deposited, but it is the later loss of brain cells that most closely predicts symptomatic decline. The timing between the appearance of toxic proteins and the brain cell loss can vary between people. Developing effective treatments remains a significant challenge; targeting treatments earlier, before symptoms, may increase success. To do this we need sensitive methods for identifying at risk individuals and tracking progression.
Our research uses advanced forms of MRI and PET brain scanning in people who have AD protein deposition but are asymptomatic, to identify and track the earliest signs of brain cell loss. We also use brain scans to identify and measure additional factors that may influence how quickly/slowly the illness progresses (i.e. the time between protein deposition, brain cell loss, and memory problems). We assess how early brain cell damage on MRI relates to new blood tests and sensitive memory tests for AD.
The research will help us diagnose people who are at very early stages of AD, and to stage disease in a way that allows identification of those most likely to benefit from new treatments.
Latest news


Dr Philip Weston
Dr Philip Weston is a Group Leader at the UK DRI at UCL. Find out more about his career and expertise on his profile page.

Research summary
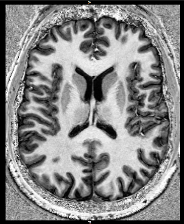
Ultra-high field MRI scan Credit: Philip Weston
Next-generation imaging biomarkers of cortical microstructure for measuring presymptomatic cortical degeneration in Alzheimer’s disease and associations with molecular pathology
In Alzheimer’s disease (AD), there is a drive towards undertaking clinical trials earlier, in the presymptomatic period, prior to significant neuronal loss. However current approaches for identifying those people who are at risk of imminent decline and tracking progression lack sensitivity, specificity and reproducibility. Additionally, there is significant variability between initial amyloid deposition and clinical decline, which is incompletely understood.
The core focus of the Weston Lab's work is neuroimaging. Their current research leverages unique and highly phenotyped human cohorts, with longitudinal data collection, to develop sensitive quantitative cortical imaging biomarkers and advance our understanding of early AD-related neurodegeneration and its link to molecular pathology. They also aim to use advanced neuroimaging to investigate additional factors which may partly mediate the rate of progression.
First, the team are undertaking multi-compartmental modelling of multi-shell diffusion MRI to measure and track early microstructural breakdown of cortical neurons, across separate but complementary cohorts, and compare with established imaging markers.
Second, the team are acquiring ultra-high field (7T) MRI and undertaking quantitive multiparameter mapping to characterize extra-neuronal cortical microstructure, including myeloarchitecture and iron deposition, at sub-millimetre resolution. Alongside this, the team are using a specially developed sequence to image the locus coeruleus (LC) – the brain primary noradrenergic output – which is affected very early be AD pathology and may represent a potential early imaging biomarker.
Thirdly, the team assess associations between these different quantitative imaging measures and biomarkers of the core AD pathologies amyloid and tau, as well as fluid measures of neuroinflammation.
An additional theme is to build on our previous work in novel cognitive testing methodology and harness new digital technology to develop a smartphone-based test of accelerated long-term forgetting (ALF). Also, in the same people, the team will work with DRI collaborators to undertake digital at-home sleep monitoring, to detect early AD-related changes and assess influence on memory.
Specific objectives include to:
- Assess the timing and nature of early microstructural change in AD, and determine how it can be optimally assessed in-vivo.
- Use 7T quantitative MRI to detect specific additional pathological processes, include iron deposition, myelin breakdown, and change in LC integrity and assess their influence on progression
- Examine the association between markers of specific neuronal microstructural changes and tau deposition.
- Develop and investigate the use of digital biomarkers of memory consolidation and sleep in presymptomatic AD.
Key publications
Vacancies
Collaborators






Lab funders
Thank you to all those who support the Weston Lab!