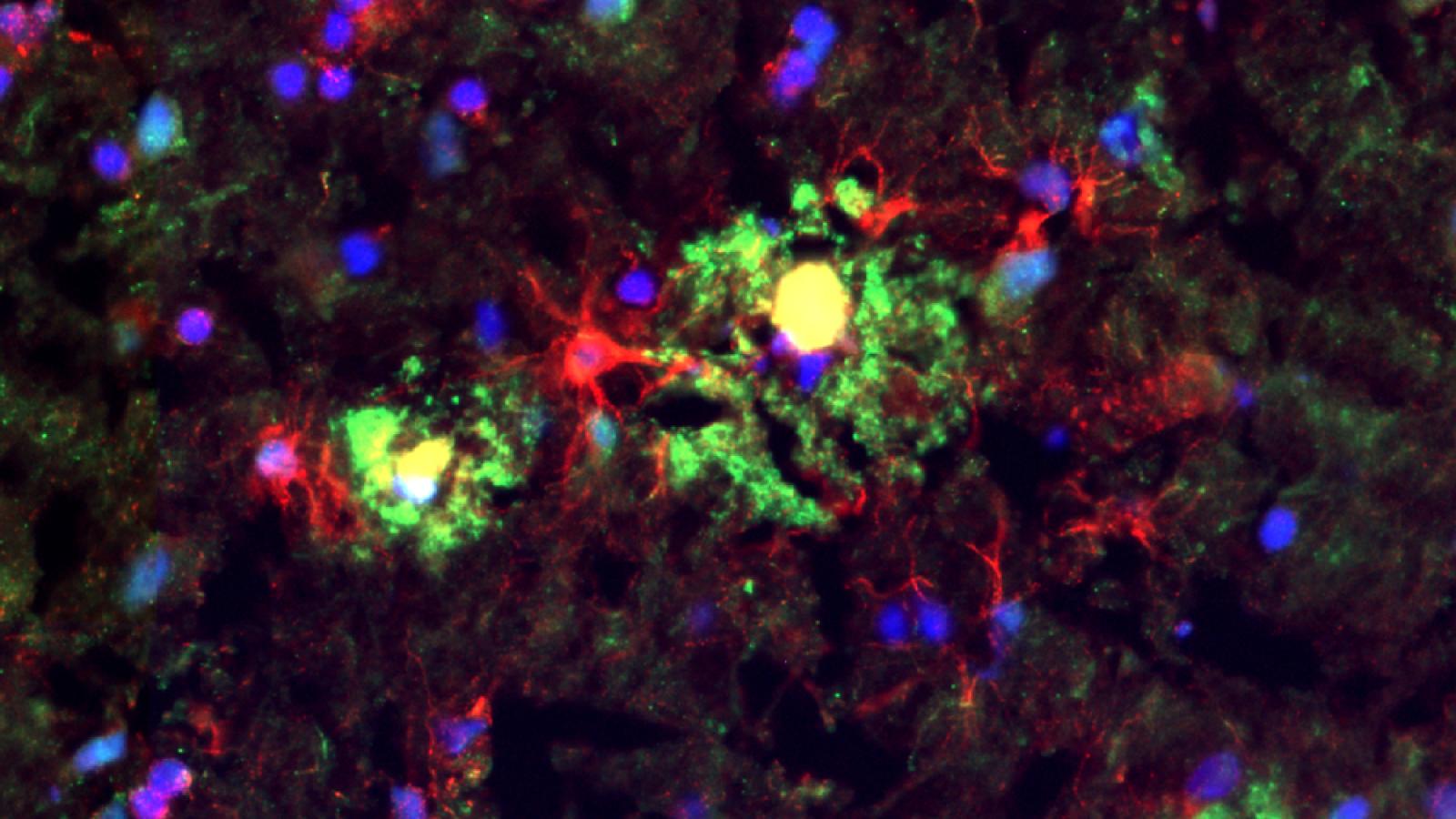
Preventing "hole-punching" activity in cells
Complement is a system of proteins in blood that exists to counter bacterial infections, either by directly killing bacteria or provoking white blood cells to eat them. The direct-killing part of complement is a protein complex called MAC which bursts bacteria and human cells alike by poking holes in their surfaces like a pinprick in a balloon. Leaking cells cause lots of inflammation, which is implicated in many diseases, including Alzheimer’s.
The Zelek Lab are testing the idea that overactivity in MAC and the inflammation it causes is an important driver of Alzheimer’s disease. The team's research involves developing new methods of targeting MAC in ways that will prevent the "hole-punching” activity that is most harmful to our own cells and the most inflammatory, while leaving intact the important roles of complement in killing bacteria. As part of this, the Zelek Lab are generating MAC-blocking drugs capable of entering the brain that can stop MAC-driven brain inflammation. The team are using test-tube models of the barrier between the blood and brain, to develop these agents and select the best ones to provide proof-of-concept for their use in Alzheimer’s, by testing them in appropriate animal models, and laying the groundwork for future human therapies.
Latest news


Dr Wioleta Zelek
Dr Wioleta Zelek is an Emerging Leader at the UK DRI at Cardiff. Find out more about her career and expertise on her profile page.

Research summary
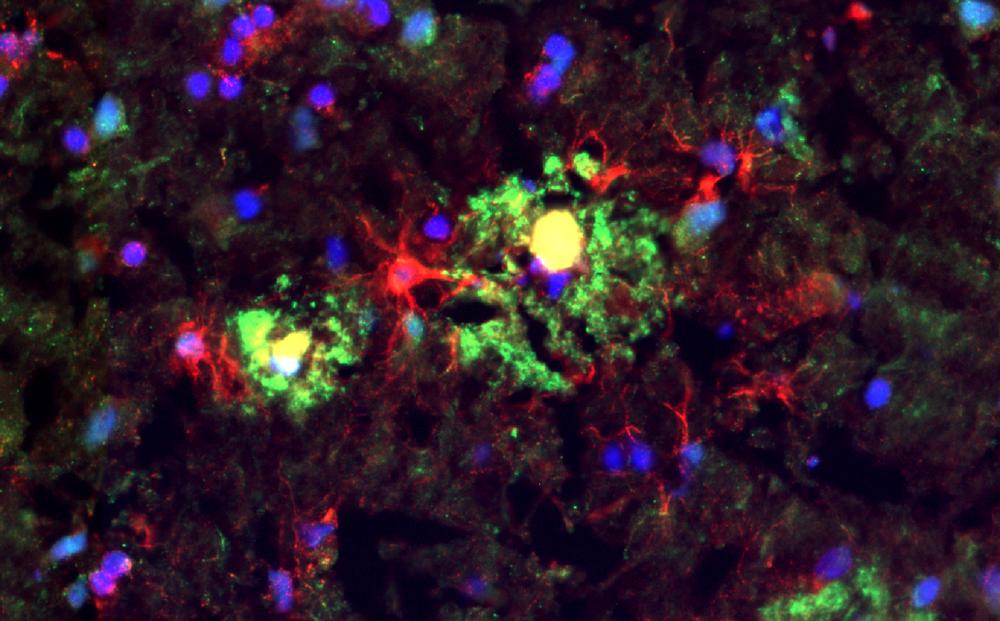
Complement receptor 1 (CR1) is found on microglia and astrocytes (red) and amyloid-beta plaques (green/yellow) in the Alzheimer's disease brain. Credit: Lewis Watkins, Zelek/Morgan Lab
Targeting novel anti-complement drugs to the brain for therapy of dementia
Complement is a central component of innate immunity, a network of plasma proteins interacting to provide defence against infection and efficient removal of dead cells and debris. Because of its complexity and destructive character, complement is tightly regulated by numerous fluid-phase and membrane regulators. Activation of complement initiates formation of enzymes, termed convertases. These include the C5 convertase, which cleaves C5 into C5a and C5b, the latter seeding formation of the lytic membrane attack complex (MAC), the subject of Dr Wioleta Zelek’s research.
Critically, genetic and epidemiological studies, cerebrospinal fluid and plasma biomarker measurements and pathological analysis have all implicated complement as a driver of pathology in Alzheimer’s disease and other dementias. To test this, Dr Zelek will develop efficient, blood-brain barrier-penetrant inhibitors. This research will lay the foundations for developing novel, transformative therapies targeting brain inflammation, providing better ways of treating Alzheimer’s and other dementias with resultant positive impact on human health.
Main objectives and research goals:
- Making small fragments of MAC-inhibitory antibodies and testing their blood-brain barrier penetrance in vitro.
- Exploring new ways of delivery of antibodies, antibody fragments and small molecules into the brain.
- Identifying the MAC binding sites of the antibody fragments to enable the design and screening of small molecule MAC inhibitors.
- After confirming that the inhibitors can cross in vitro blood-brain barrier models, the best agents will be tested for in vivo blood-brain barrier penetrance and impact on disease in Alzheimer’s disease models.
Key publications
Vacancies
Lab members
- Rob Byrne (Postdoctoral researcher)
- Dr Kok Yung Lee (Research Associate - joint with Paul Morgan)
- Jack Reddaway (Research Associate - joint with Paul Morgan)
- Dr Gareth Fenn (Research Associate - joint with Paul Morgan)
- Kirsten Baillie (Research technician)
- Emily Pockett (Research assistant)
- Rebekah Cooke (PhD student)
- Zhizhong Yang (PhD student)
- Matthew Bright (PhD student)
- Lara Lennon (PhD student)
- Laura Nicholls (PhD student)
- Taine Baggott (PTY student)
- Ruby Evans (Student)
- Ava Burge (Student)
- Mariana Flores Castelan (Technician)
Collaborators






Lab funders
Thank you to all those who support the Zelek Lab!