Last year, a new medication, lecanemab, hit the headlines when Phase III trial results confirmed that it had become the first of its kind to slow cognitive decline in people with Alzheimer’s disease. The findings not only appear to validate the long-standing amyloid-cascade hypothesis in the disease, but also seemingly support the theory of ‘right drug, right patient, right time’, in which targeted selection of participants at the early stages of the disease - those with mild cognitive impairment due to Alzheimer’s in this trial - is thought to offer the drug the best chance of disrupting disease progression before extensive neuronal loss occurs. Intervening before the onset of even the most subtle of symptoms has the potential for even greater benefit.
But if an individual is at the earliest stages of Alzheimer’s, and does not present with any symptoms of the disease, how can they be accurately diagnosed and identified as prime candidates for clinical trials? This is where the field looks to ‘biomarkers’ - biological measures or ‘signposts’ that aid in the identification and monitoring of a person’s disease.
Amyloid beta is the protein implicated in the amyloid-cascade hypothesis, and its accumulation in Alzheimer’s is used as an early biomarker either through positive cerebrospinal fluid (CSF) samples or a Positron emission tomography (PET) brain imaging scan. However, the build-up of amyloid beta plaques in the brain occurs many years to decades before people have symptoms. If a substantial number of amyloid-positive trial participants do not go on to develop symptoms of Alzheimer’s, this can have a major impact when verifying the effectiveness of a new drug.
With most cognitive measures declining too late in disease to be useful, and the presence of amyloid beta alone proving an unreliable predictor of symptom onset, there is an unmet need for a new type of biomarker that can accurately diagnose the earliest stages of Alzheimer’s. This grand challenge is the subject of a new £1.7 million investment from UK DRI led by UKRI Future Leader Fellow and UK DRI Group Leader at UCL, Dr Marc Aurel Busche.
In this highly collaborative project, involving multiple experts from outside the dementia field, the team aims to develop a functional biomarker that measures subtle changes to the brain’s circuitry at an early stage of Alzheimer’s, and before the tipping point into irreversible cognitive decline.
"I think a good analogy would be cardiology – if you were concerned for the cardiovascular health of a patient, you would not only take a blood test to measure something like cholesterol, you would also perform electro- and echocardiograms to gain a more accurate picture of the state of the heart at work. As a clinician, I may receive the results of abnormal fluid biomarkers suggesting the presence of Alzheimer’s pathology in a patient, but I can never be sure if they are one of the 30% of people who will not go on to develop cognitive symptoms. What we need is a reliable readout that confirms there are early pathophysiological changes to the brain, one which more accurately predicts and tracks the development of disease and symptoms.”
Dr Busche’s pioneering research investigates the impact of abnormal proteins like amyloid beta on the brain’s cells and circuitry, using innovative mouse models and cutting-edge techniques that are custom-built in his laboratory. Notably, he has shown that amyloid-beta causes hyperexcitability in the brain’s neuronal networks early in Alzheimer’s, leading to impaired learning and memory.
Grand Challenge Award
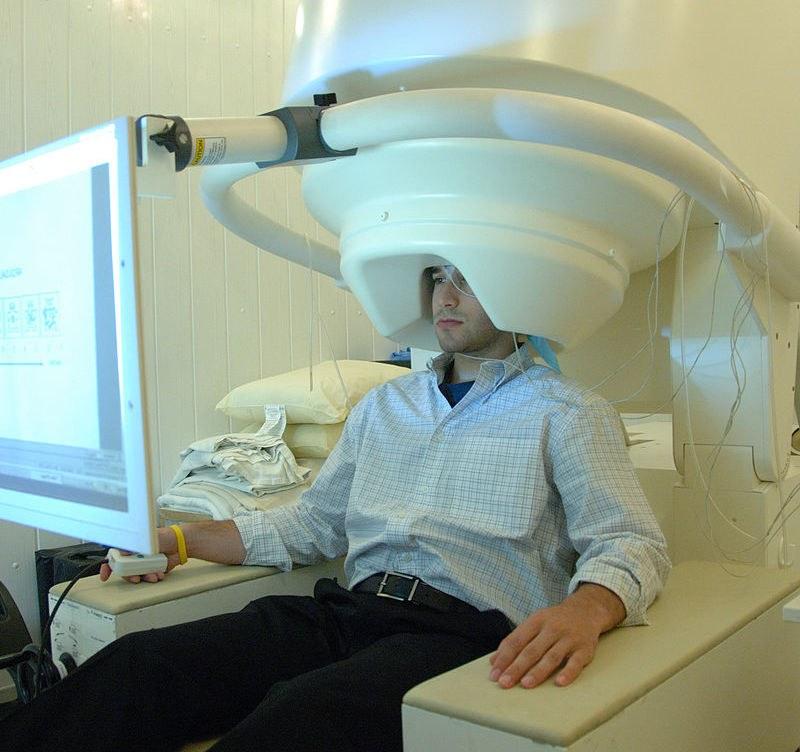
The team will harness whole-brain magnetoencephalography (MEG) and machine learning to explore how different forms of neural replay are affected during disease progression
In the search for an effective functional biomarker for Alzheimer’s, Dr Busche has teamed up with 2017 Brain Prize winner Prof Ray Dolan, world-leading psychiatrist and Professor of Neuropsychiatry at UCL. It is Prof Dolan’s recent work on ‘neural replay’ that piqued Dr Busche’s interest.
Neural replay is now a well understood phenomenon important in learning and spatial memory across species. When someone is navigating, the brain’s ‘place cells’ show a sequence of neural activation. During sleep and certain awake periods, these cells fire again in a related, but time compressed, sequence. The theory is that this replay strengthens the synapses, or connections, between the cells, consolidating the memory of where someone was or the path they took. Its function is even thought to help with future navigation, and other cognitive processes.
A breakthrough in the field came from Prof Dolan’s lab, when the team showed for the first time that neural replay could be measured in humans using a brain scanning technique known as magnetoencephalography (MEG). Prof Dolan explains why neural replay has the potential to be a good functional biomarker for Alzheimer’s:
“Neural replay originates in the place cells found in the hippocampus. This is a region of the brain important for learning and memory, and crucially is impacted very early in Alzheimer’s disease.”
The team therefore hypothesise that the emergence of abnormal Alzheimer’s-related proteins in the hippocampus will disrupt neural replay. This is anticipated to be detected early on in the disease process, especially compared to cognitive processes taking place in other brain regions, and providing the early functional readout required.
During human studies, new assessment tasks will be developed, and machine learning approaches harnessed, to determine how different forms of neural replay are affected during disease progression. This will be tested during the earliest stages of Alzheimer’s disease, through a collaboration with UK DRI Group Leader at UCL, Prof Nick Fox, and senior clinical research fellow and honorary consultant neurologist, Dr Natalie Ryan.
The team at UCL’s Dementia Research Centre have access to two special cohorts of research participants. Firstly, individuals with the more common sporadic form of the disease who are just beginning to show memory problems. Even by this stage, the team believes neural replay may already be impaired in the hippocampus and provide an important proof-of-concept for the MEG technology.
However, the ultimate aim of the project is to detect subtle circuitry changes before cognitive symptoms appear. The researchers will therefore also carry out investigations in people who have rare genetic mutations leading to an early onset form of the disease. These individuals are well characterised by Prof Fox’s team, and also allow comparison of MEG results with other biomarkers such as amyloid beta measurements in the blood or cerebrospinal fluid (CSF).
Special cohorts of research participants from UCL’s Dementia Research Centre will be involved in human studies
Animal behaviours often do not translate well to humans and this has proven a major issue in discovery research. Neural replay, on the other hand, is a phenomenon conserved between rodents and people, and it is thus of significant translational value, enabling findings from the lab and clinic to be interpreted side by side and to inform each other.
Parallel to the human studies, Dr Busche and Prof David Dupret, a specialist behavioural neurophysiologist at the University of Oxford, will investigate the impact of amyloid beta and tau protein pathology on neural replay, using state-of-the-art mouse disease models. High resolution imaging, electrophysiology and complimentary behavioural tasks will allow the team to focus in on the underlying biological mechanisms that impact the brain’s circuitry, bringing greater understanding to changes seen at the human level. For Dr Busche, this is another very strong aspect of the project.
“I'm very excited and motivated. It's a unique project because of this cross-species paradigm. We're looking at a physiological signal that is present in mice and humans and that is very well understood. I think the translational potential of this project is huge, with the promise of developing something that actually changes clinical practice.”
A functional biomarker would certainly be a game changer. Used alongside other types of biomarkers, neural replay would allow clinicians to accurately diagnose Alzheimer’s earlier and therefore intervene with new treatments before significant damage occurs in the brain, as Dr Busche explains:
“Neurodegenerative conditions impact regions of the brain at different times so early and specific disruption to neural replay would allow us to pinpoint the diagnosis to Alzheimer’s. However, there remains the possibility that other hippocampal pathologies may also cause impairment so it’s important that we take the opportunity to harness a range of different functional biomarkers to improve accuracy in the clinic.”
Additionally, MEG technology is currently restricted to certain facilities and not widely available. If successful, the team will take this proof-of-concept and look to replicate the findings with smaller, cheaper devices that are already in use, or even electroencephalogram (EEG) to improve patient access.
The benefits also extend to clinical trials where accurate and reliable diagnosis would vastly improve monitoring of a drug’s impact. Beyond diagnosis and tracking, Dr Busche also predicts that there may even be potential to manipulate the brain circuit activity directly, based on their understanding of neural replay in Alzheimer’s, to modify the disease course non-invasively.
The neural replay project is due to begin in July 2023. Find out more about Dr Marc Aurel Busche's research on his UK DRI profile.
Sign up to hear about updates from both projects, and all the research highlights from the UK DRI, in our monthly newsletter – Inside Eye on UK DRI.
Article published: 20 February 2023
Banner image: Shutterstock / vs148
Image: MEG scanner with patient - National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services
Video: Courtesy of Dr Marc Aurel Busche who uses state-of-the-art technology to study interactions between toxic protein plaques (red) and blood vessels (green) in the brains of genetically-altered mice which mimic aspects of Alzheimer's disease pathology.
