Action & Reaction – Probing the response of brain cells in dementia
Alzheimer’s disease (AD) is characterised by abnormal clusters of protein in the brain and it is thought that they or the mechanisms leading to their formation, could play a role in disease progression. Individuals with an inherited form of AD often have a mutation in the gene for one of these implicated proteins – Amyloid Precursor Protein (APP) which can be cleaved into toxic Amyloid beta (Aβ) protein fragments – and therefore, many scientists believe this protein also plays a significant role in the more common non-familial and late-onset form of the disease.
Unfortunately, clinical trials targeting Aβ have proved disappointing, suggesting a more complex mechanism at play. By probing further into the genetics of people with AD, a number of further risk genes become apparent, particularly linked to the immune system and other support cells in the brain. The De Strooper Lab believes that understanding the reaction of immune and other support cells, to “agitators” such as Aβ and Tau protein, may be key to finding new disease pathways to target.
In this research programme, the De Strooper Lab investigates the downstream effects of Aβ, including the beneficial and detrimental response by brain cells - neurons as well as the many other support cells. The group utilises state-of-the-art technology to analyse individual cells as, even within the same cell type, small variations may be critical to our understanding of how the disease develops. The overall aim is to identify key cellular changes during AD, which will hopefully lead to targets for treatments.
The De Strooper Lab is co-led by Principal Staff Scientist, Dr Lorena Arancibia Carcamo.
Prof Bart De Strooper
Prof Bart De Strooper is a Group Leader at the UK DRI at UCL. Find out more about his career and expertise on his profile page.

Research summary
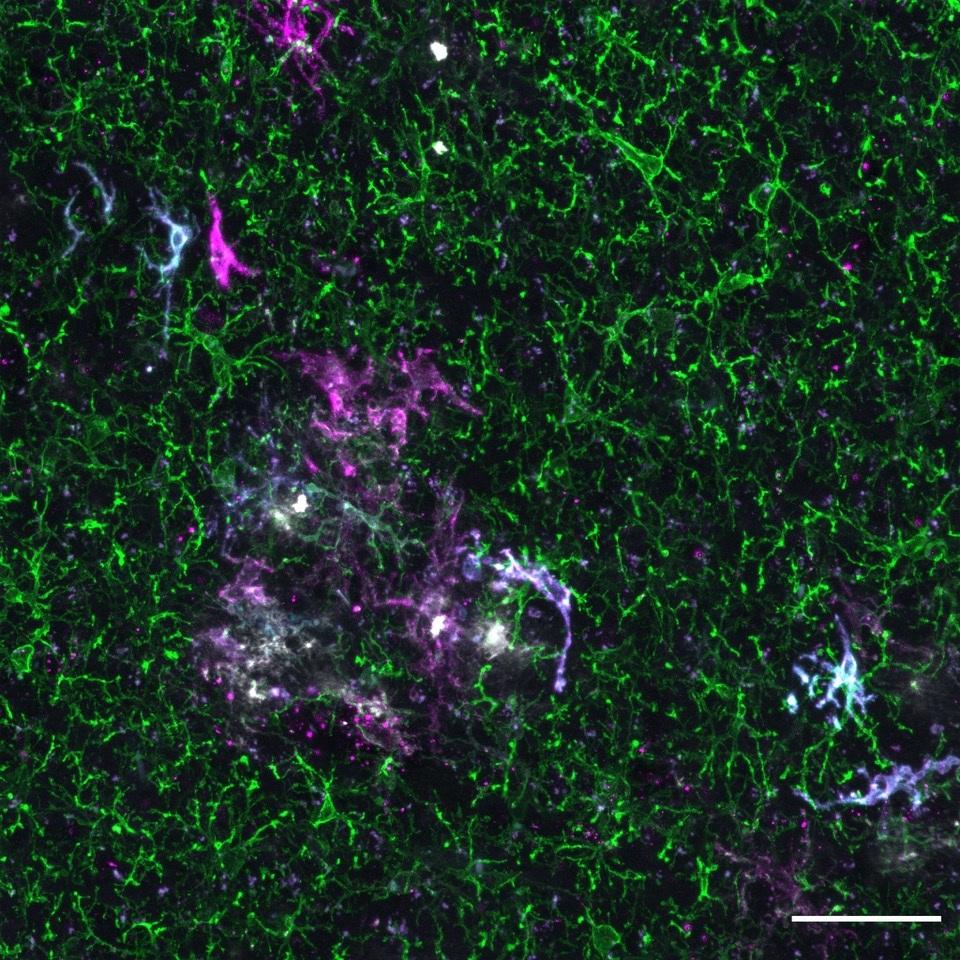
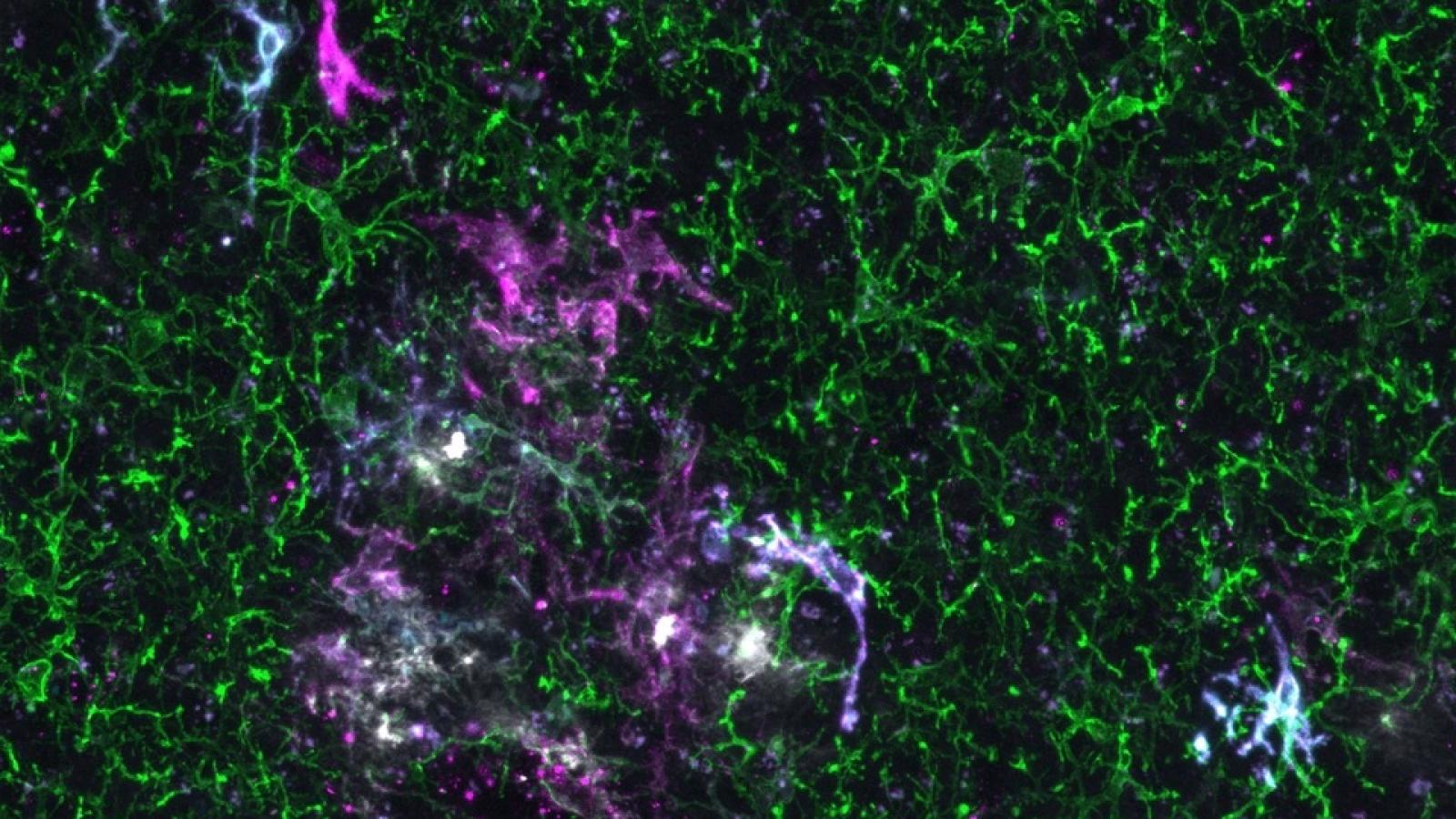
Xenografted human microglia display diverse transcriptomic states in response to Alzheimer’s disease-related amyloid-β pathology. Mancuso et al. Nature Neuroscience, 2024.
Investigating the cellular reaction to amyloid beta and Tau
Alzheimer’s disease (AD) is characterised by the build-up of abnormal proteins and widespread neurodegeneration. In individuals with the familial form of the condition, mutations in the amyloid precursor protein (APP), and proteins involved in processing of APP, such as gamma & beta secretase, cause the production of longer amyloidogenic amyloid beta (Aβ) peptides – thought to be the primary cause of AD. The amyloid-cascade hypothesis has been the prevailing theory for disease pathogenesis, however, results from clinical trials that target components of this pathway have proved disappointing. However, we have much to learn from their ‘failures’, and we must also address the heterogeneity and stages of different disease types.
There is now a greater appreciation within the field as to the vast complexity of AD and other neurodegenerative disorders. To decipher disease mechanisms, the De Strooper Lab's research has interrogated the genetics of both the familial and sporadic forms of the condition. Genome wide association studies (GWAS) consistently flag microglia and astroglia besides the amyloid pathway and Bart believes that targeting the cellular reaction to the Aβ may prove more effective than the protein itself.
In this programme at the UK DRI at UCL, Bart and his team focus on determining the downstream effects of Aβ, from the biochemical to cellular phase, and deciphering both the beneficial and detrimental response of brain cells such as microglia, astrocytes and oligodendrocytes. Recent evidence suggests that there are thousands of risk genes that confer a low risk for the development of AD. Bart is particularly interested in these previously overlooked genes as, when combined with other risk genes, and/or in aggravating conditions e.g. ageing or Aβ deposition, they may have a greater detrimental impact and lower the threshold for disease onset. This may be a critical aspect of sporadic forms of AD, where a strong genetic component, e.g. mutations in APP, is not observed.
Although there are numerous animal models aiming to mimic AD, none of them recapitulate all characteristics and pathologies of the condition fully. Furthermore, while mouse and human immune cells, such as microglia, have similar transcriptional signatures, this partially changes with age. Recent evidence also suggests the signatures from human microglia derived from AD brains, differ dramatically from mouse microglia in disease conditions. In attempts to overcome these issues, the De Strooper Lab has recently developed a chimeric model by injecting human microglia derived from embryonic stem cells into brains of young mice. Eight weeks later, the human cells had taken on a seemingly normal, ramified appearance and distributed across the mouse brain in a classic microglial tiling pattern. Crucially, it appears they also keep their human transcriptomic signature. This model, therefore, has the potential to revolutionise microglia studies, yielding results more accurately reflecting the human condition.
The transcriptomic work carried out thus far has highlighted the extensive heterogeneity across microglial cell phenotypes, however, questions still remain as to the functional consequences in a disease context. By building collaborations and drawing on expertise in areas such as proteomics and lipidomics, the team brings together this knowledge to push toward therapeutic development for the clinic.
Main objectives and research goals:
- Elucidate the downstream effects of Aβ with respect to cellular response.
- Utilise the novel humanised chimeric models to study the role of human microglia, astroglia, human neurons and oligodendrocytes in the cellular phase of Alzheimer’s Disease.
- Explore low-risk genetic factors that may reveal cellular components and pathways important in AD mechanism.
- Identify targets for early therapeutic intervention.
Key publications
Dr Lorena Arancibia Carcamo
The De Strooper Lab is co-led by Principal Staff Scientist, Dr Lorena Arancibia Carcamo. Learn more about her career and expertise on her profile page.

Lab members
- Dr Lorena Arancibia Carcamo (Principal Staff Scientist)
- Dr Marc Fiers (Senior Postdoctoral Researcher)
- Dr Amy Lloyd (Research Fellow based at the University of Dundee)
- Dr Disha Shah (Postdoctoral Researcher)
- Emily Blackburn (Postdoctoral Researcher)
- Emma Davis (Research Assistant)
- Millie Thackray (PhD Student)
- Perlina Desai (PhD Student)
- Brendan Hajar (PhD Student)
- Amer Townsend (Senior Technician)
- Carlo Zanetti (Research Assistant)
- Alexia Vereertbrugghen (Research Assistant)
Vacancies
Collaborators








Lab funders
Thank you to all those who support the De Strooper Lab!