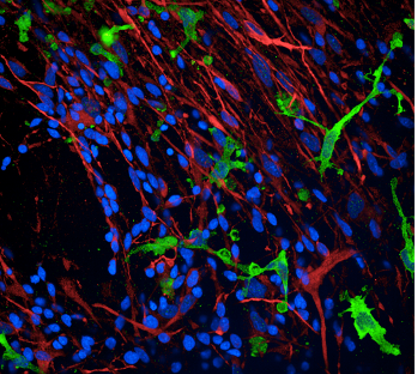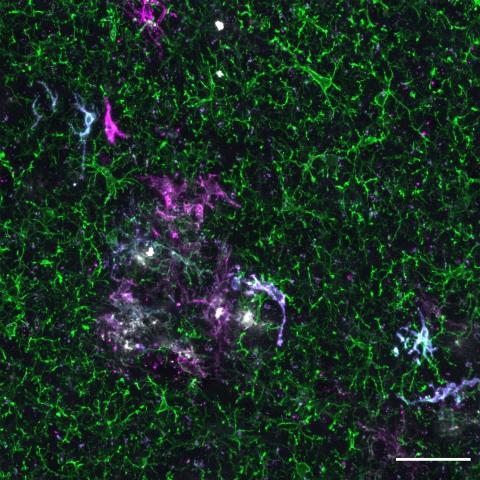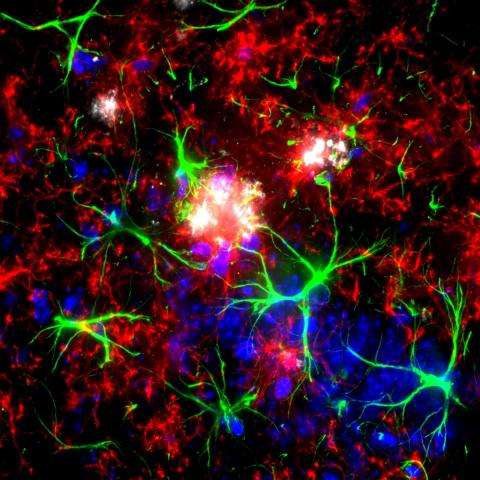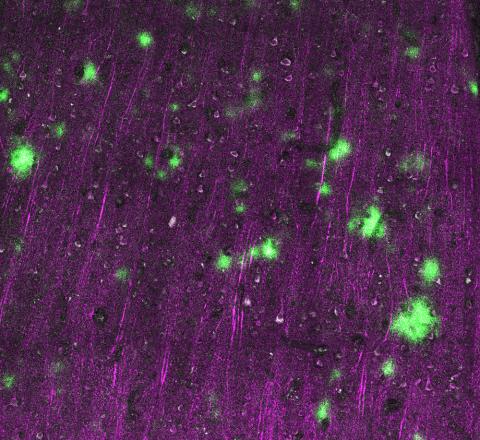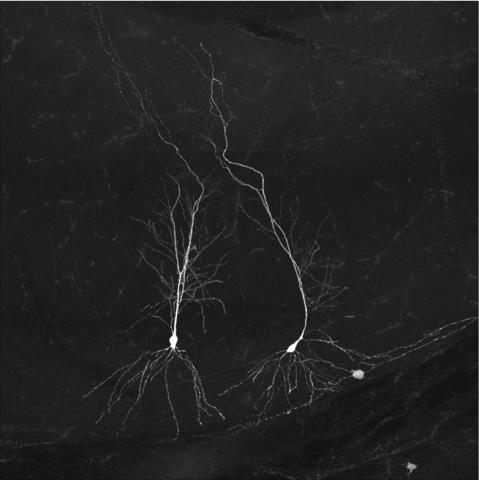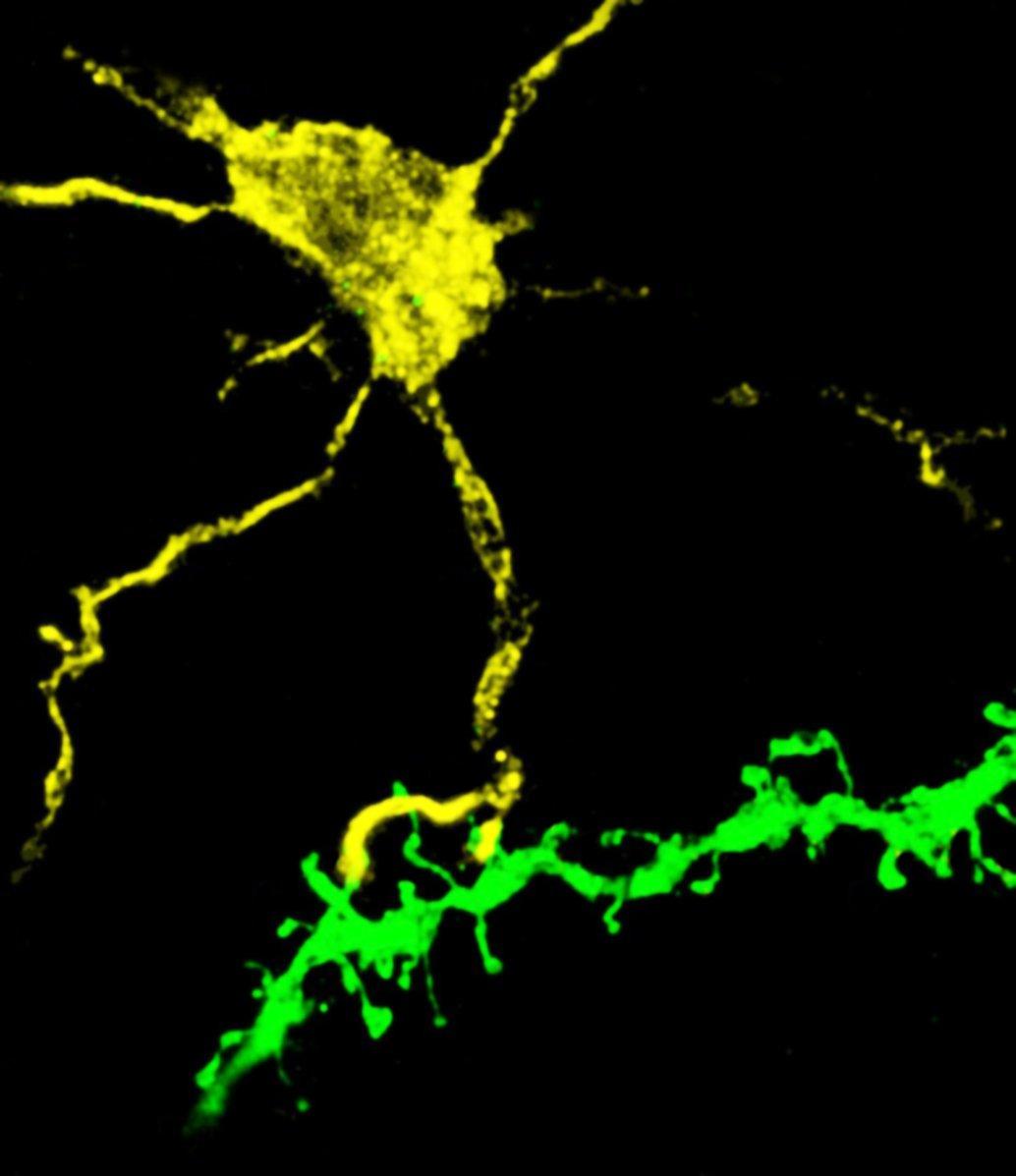
Confocal image of the brain section from a Thy1-eGFP mouse. The image depicts the interaction between the process of microglia and a selective subset of dendritic spines. Credit: Xingjian Wang (Barnes Lab)
Synapse dysfunction and loss are fundamental to the pathophysiology of neurodegenerative disorders and correlate strongly with poor cognitive outcome. One of the earliest signs of Alzheimer’s disease is changes to electrical activity patterns within regions of the brain that are key for learning and memory. Although the molecular causes behind this abnormal brain activity are not yet understood, they may contribute to the gradual loss of synapses between neurons. Our understanding of the processes that mediate these changes in electrical activity, how it leads to synapse dysfunction and elimination, and the repair and compensatory mechanisms within the neuron to counter and compensate for the synapse loss remain largely unexplored.
Synapses are highly complex structures composed of thousands of distinct proteins, which makes characterisation challenging. Synapse types have traditionally been discriminated based on neurotransmitter molecules, but it is now clear that protein composition exhibits deeper diversity within traditional neurotransmitter types. Such diversity is further complicated by additional factors, such as brain-state dependent changes in synaptic activity, regional susceptibility to disease pathology and interplay with non-neuronal cell-types. To date, only a handful of studies have attempted to determine individual elements of synaptic vulnerability, with many focusing on features such as molecular composition, genetic signatures or pathophysiological activity – all in isolation.
The UK DRI is ideally placed to address the links in mechanisms underlying selective synapse vulnerability by bringing together expertise studying:
- molecular composition of synapses in health and disease states
- the role of toxic aggregates on synapse function
- the processes that mediate synapse loss, including the role of the microglia in this process
- the transsynaptic spread of toxic aggregates
- the effects of the toxic aggregates at a network and behavioural level
- brain-state dependent changes in synaptic activity
We are also exploring opportunities to translate our findings through non-invasive neuromodulatory interventions and developing biomarkers that measure synapse degeneration.