Info
of Alzheimer's cases are sporadic, or late-onset
of the risk of developing sporadic Alzheimer's is genetic
Understanding the genetics of Alzheimer's
Alzheimer’s disease has huge personal and economic costs, and as yet there are no effective treatments. The majority (more than 99%) of Alzheimer's cases are considered to have no clear underlying cause and are known as ‘sporadic’. However, 60-80% of the risk of developing sporadic - also known as late onset - Alzheimer's, is known to be genetic. The lack of understanding of the genetics of this condition is the major bottle neck to producing new and effective therapies.
Multiple large genetic studies have identified small changes more common in people who develop late-onset Alzheimer's than in healthy individuals. Some of these changes sit within a specific pathway used by the cells in the brain, known as the endocytic pathway. The endocytic pathway is responsible for recycling and clearing waste from our cells, as well as maintaining healthy cells.
The work of the Connor-Robson Lab focuses on understanding how these genes, associated to both late-onset Alzheimer's and the endocytic pathway, contribute to the cellular changes leading to the onset of Alzheimer's, with the hope of developing novel therapies.
Latest news
Dr Natalie Connor-Robson
Dr Natalie Connor-Robson is an Emerging Leader at the UK DRI at Cardiff. Find out more about her career and expertise on her profile page.

Research summary
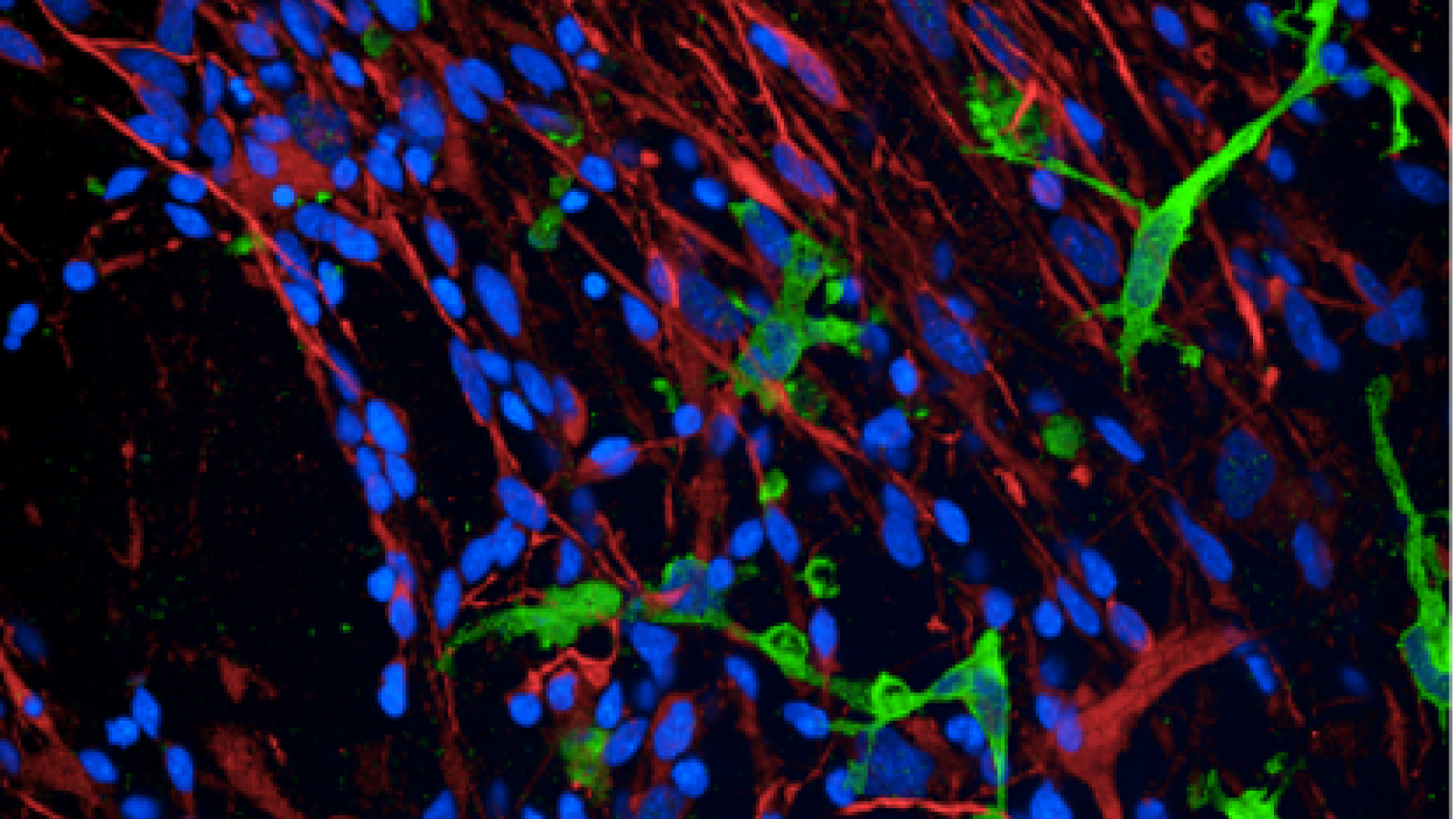
Amyloid Beta protein is phagocytosed by iPSC-derived microglia. Credit: Natalie Connor-Robson
Understanding the role of the endosomal pathway in late onset Alzheimer’s disease
Research into Alzheimer's has concentrated on monogenic forms of the disease which account for <1% of cases. Across the remaining >99% of cases, between 60-80% of risk for developing sporadic Alzheimer's is still considered to be genetic. The Connor-Robson Lab uses a genetics-led approach to gain vital insights into our understanding of the pathogenesis of Alzheimer's with the aim of using this information to develop effective therapeutics for the most common sporadic form of the disease.
Multiple genome-wide association studies have independently identified single nucleotide polymorphisms (SNPs) for late-onset Alzheimer's. Pathway analysis has shown that a proportion of these risk genes cluster into the endocytic pathway including BIN1 and PICALM, the two most significant risk genes after APOE, CD2AP and SORL1. The endocytic pathway is one of the most strongly implicated biological pathways in late-onset Alzheimer's through both genetic and pathological evidence with endocytic dysfunction being an early and highly consistent pathological feature in post-mortem Alzheimer's brains.
The Connor-Robson Lab uses iPSC-based models to understand the molecular mechanisms underlying the endocytic late-onset Alzheimer's-associated genetic changes in both neuronal and glail cells. Natalie's team have developed a multiple novel iPSC lines through CRISPR gene editing and through reprogramming of patient samples. As well as assessing single risk genes, they also investigate how multiple endocytic risk genes come together in the development of late-onset Alzheimer's using a novel endocytic polygenic risk score. The team aim to study these genetic alterations in different cells types to better understand cell type specific phenotypes and identify key points and processes in the endocytic pathway that can be targeted by therapeutics.
Key publications
Vacancies
Lab members
- Sri Goberdhan (Senior Technichian)
- Dr Mizuki Morisaki (Research Associate)
- Matilda Kakengi (PhD student)
Collaborators
















Lab funders
Thank you to all those who support the Connor-Robson Lab!