Info
Investigating how complement contributes to Alzheimer's disease development
Alzheimer’s disease (AD) is the most common cause of dementia, accounting for around two-thirds of cases in older people. Although researchers don’t yet fully understand what triggers the disease, the build-up of toxic protein aggregates or 'clumps' in the brain is thought to be involved in driving its progression – causing damage and death to neurons.
For many years, the brain was thought to be ‘sealed’ from the rest of the body’s immune system by the blood-brain-barrier – preventing the entry of immune cells and molecules. However, scientists now appreciate various immune processes do operate within the brain and two-way communication, between here and the rest of the body, does occur. For instance, key immune proteins known as ‘complement’ are activated in the brains of people with AD. But scientists don’t fully understand the consequences - on one hand, this may help with waste clearance and prevent the build-up of toxic proteins while on the other, it could promote inflammatory responses that help drive disease progression.
The Morgan Lab is investigating exactly how complement contributes to AD development. Their results will support the development of new tests that can help identify people with increased risk of developing AD or detect the disease early when interventions may be more effective. It will also inform the discovery of new drugs that can prevent or treat the condition by targeting complement.
Latest news


Prof B. Paul Morgan
Prof B. Paul Morgan is a Group Leader at the UK DRI at Cardiff. Find out more about his career and expertise on his profile page.

Research summary
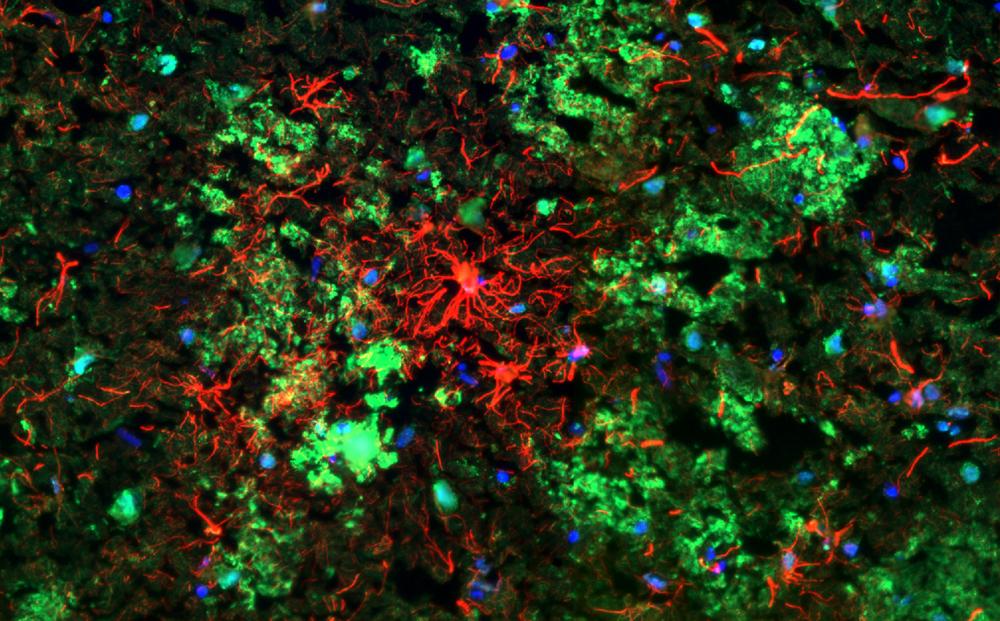
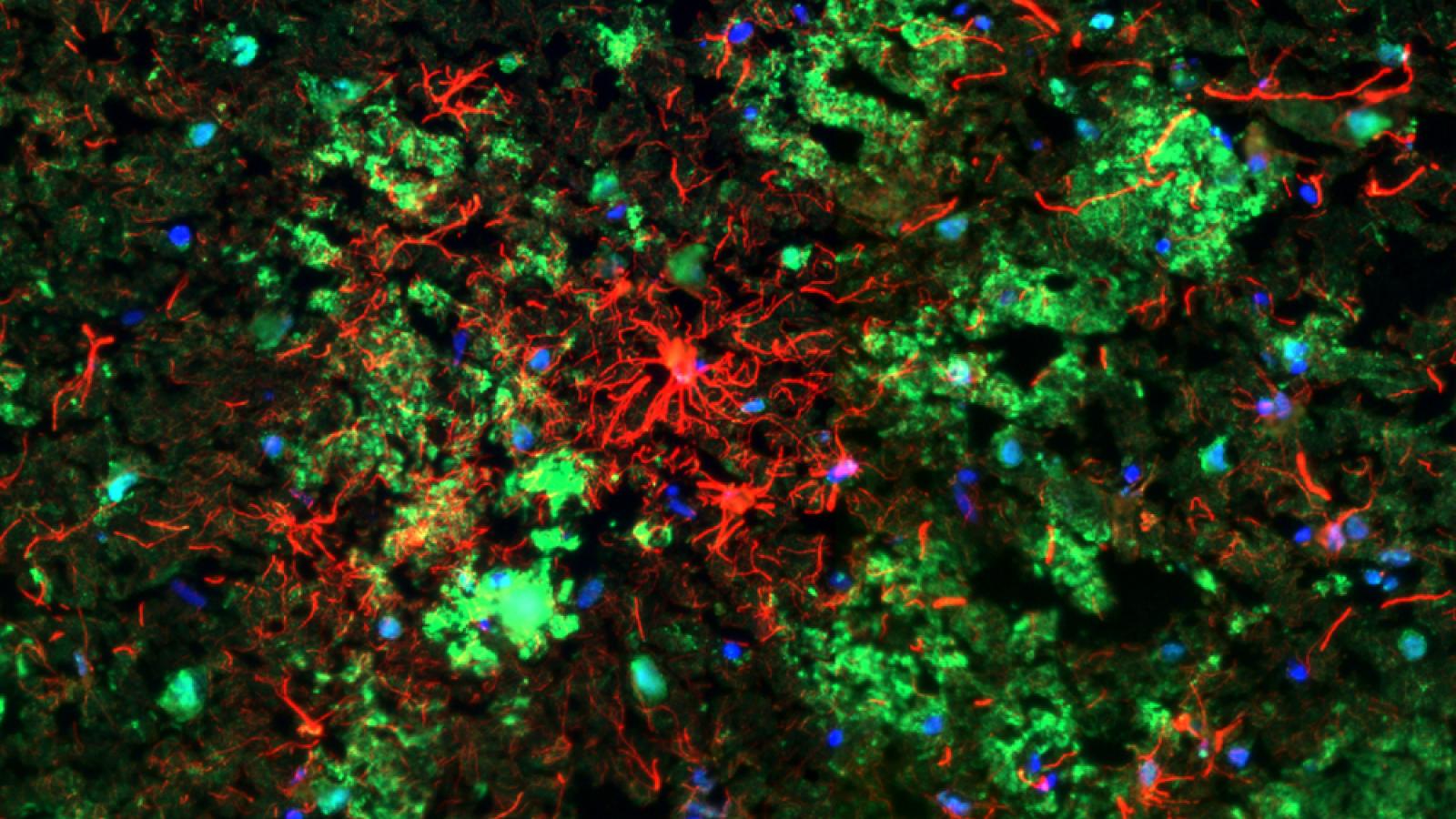
CSMD1 (red)-positive astrocytes surrounding and being surrounded by numerous amyloid-beta plaques (green) in the Alzheimer's disease brain. Credit: Lewis Watkins, Zelek/Morgan Lab
Complement – a help or hindrance in Alzheimer’s disease?
Gene discovery has implicated innate immunity, inflammation and the complement system as drivers of pathology in Alzheimer’s disease (AD). The involvement of complement is further supported by a large and growing body of evidence from animal studies, human biomarker and immunopathology findings and molecular pathway analyses.
Prof Paul Morgan’s recent work has identified plasma complement biomarkers predictive of AD; showing that plasma levels of complement proteins and activation markers in mild cognitively impaired (MCI) individuals are highly predictive of progression to AD, suggesting that complement is involved early in the disease process. These same complement biomarkers mirrored the polygenic risk score for AD, linking genetics to proteins. Complement also overlaps with and strongly influences other gene-implicated pathways such as phagocytosis, membrane turnover and debris removal, placing it at a nexus in the disease process and flagging as a potential target for therapeutic intervention.
Understanding precisely how complement contributes to the disease process in AD, a prerequisite for effective targeting, has been challenging. A simplistic interpretation might be that complement activation drives pathology by amplifying inflammation, activating glia and exacerbating neuronal damage; however, there is compelling evidence that complement activation can, in some circumstances, reduce or reverse AD pathology – the double-edged sword. For example, strategies that aim to enhance amyloid clearance using antibodies or immunisation rely on complement activation to opsonise amyloid for phagocytic removal, while in mouse AD models, complement C3 deficiency exacerbates disease in some and ameliorates in others.
This ambitious programme from the Morgan Lab builds on recent findings and local strengths in gene discovery, brain imaging and drug development to achieve an understanding of the roles of complement at different stages of disease progression in AD models and patients. This detailed understanding will support the discovery of predictive biomarkers and guide the development of safe and effective anti-complement drugs for the prevention and therapy of AD.
Main objectives and research goals:
- To explore the impact of AD-associated complement gene polymorphisms. Identifying mechanisms by which risk variants impact complement component expression and/or function and predispose to AD.
- To analyse the ex vivo effects of complement on neuronal/synaptic health and phagocytosis. Characterisation of the effects of complement activation and regulation on synapses using cultured murine synaptosomes and synaptoneurosomes.
- To carry out an in vivo characterisation of complement roles in disease development. Identifying roles of complement in driving pathology in AD mouse models using a combination of gene deletion and pharmacological interventions.
Key publications
Lab members
- Dr Wioleta Zelek (Emerging Leader)
- Dr Sarah Carpanini (Postdoctoral Researcher)
- Dr Nikoleta Daskoulidou (Postdoctoral Researcher)
- Dr Timothy Hughes (Postdoctoral Researcher)
- Dr Lewis Watkins (Postdoctoral Researcher)
- Dr William Macintosh-Smith (Research Associate)
- Yanling Hu (Research Associate)
- Dr Gareth Fenn (Research Associate - joint with Wioleta Zelek)
- Jack Reddaway (Research Associate - joint with Wioleta Zelek)
- Dr Kok Yung Lee (Research Associate - joint with Wioleta Zelek)
- Michelle Doyle (Technician)
- Vinira Wijesurija (Technician)
- Taine Baggott (Technician)
- Jade Gregory (PhD Student)
- Joel Punter (PTY Student)
- He Zhao (PhD Student)
Vacancies
Collaborators



Lab funders
Thank you to all those who support the Morgan Lab!