A ‘starring’ role for astrocytes in dementia?
The Hardingham Lab is studying star-shaped cells called astrocytes to better understand their role in helping maintain a healthy brain over many decades – and how they contribute to neurodegenerative diseases. Boosting our understanding of the biology and function of these previously-overlooked immune cells could lead to new ways to treat dementia in the future.
Neurons receive the most attention out of all brain cell types and are the spotlight of dementia research around the world – particularly as their death is the hallmark of neurodegenerative diseases. However, they don’t work alone – they are one component of a complex wider brain network that also includes billions of other cells, including astrocytes.
Over the past few decades, scientists have started to uncover a number of crucial roles for astrocytes – not only do they provide physical structure to the brain and vital support to neurons but are also key players in the immune system, help maintain brain blood vessels (blood-brain-barrier), provide energy to neurons, and also have a role in waste clearance. Increasing our knowledge about the biology of these cells, how they contribute to the wider brain system and identifying key changes involved in neurodegenerative diseases will open the door for the development of effective new therapies.
Latest news

Prof Giles Hardingham
Prof Giles Hardingham is a Group Leader and Centre Director at the UK DRI at Edinburgh. Find out more about his career and expertise on his profile page.

Research summary
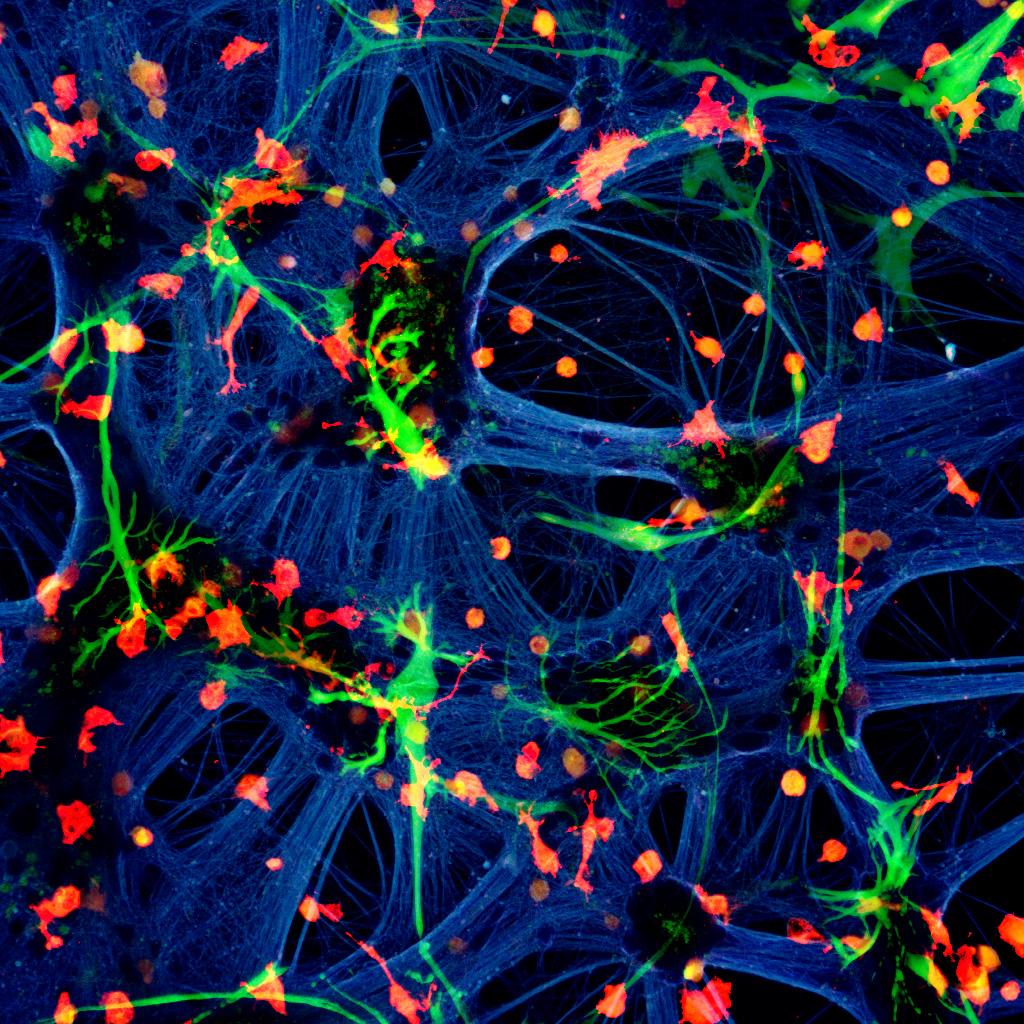
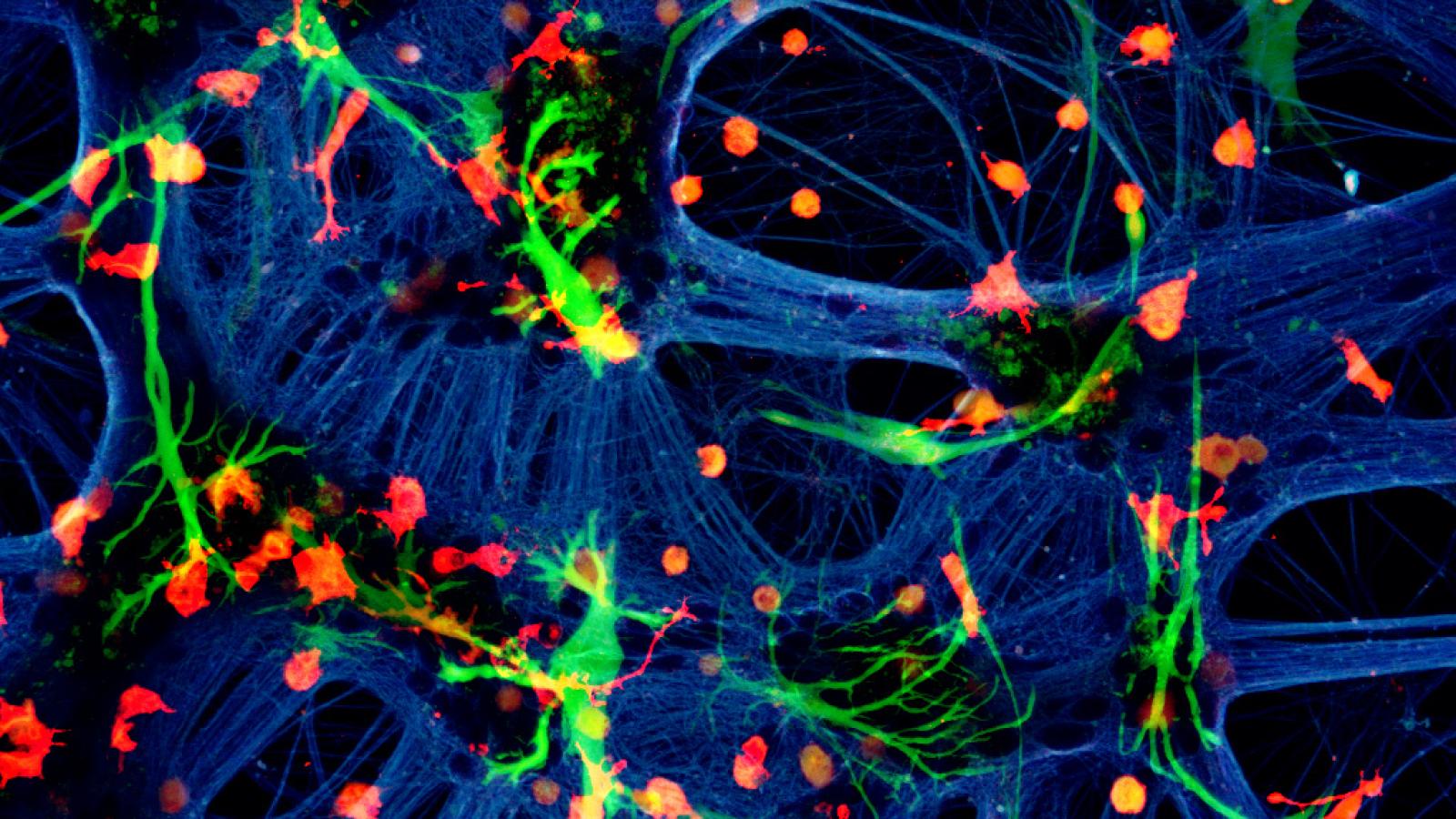
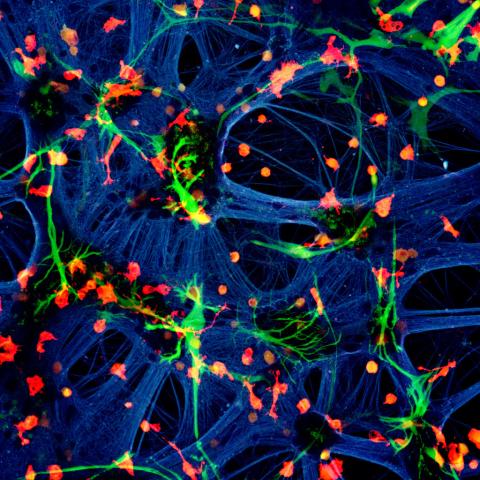
A mixed species co-culture with mouse neurones (blue – beta-tubulin), human astrocytes (green – GFAP) and rat microglia (red – Iba1). Credit: Dr Paul Baxter, Qiu & Hardingham Labs
Astrocytes as an upstream modulator and downstream effector of neurodegenerative pathology
This UK DRI programme, led by Prof Giles Hardingham, is focussing on astrocyte interactions with neurons and microglia in health and disease, the mechanisms of astrocyte-mediated homeostatic support, as well as the consequences of its disruption in neurodegenerative disorders, and their potential as a target for disease-modifying therapies.
There is a need to fully profile the astrocytic changes in Alzheimer’s disease (AD) and other neurodegenerative disorders, to pinpoint any functional deficits that arise, and the gene expression profiles that underlie them. Equally important is to understand how astrocytes tune their homeostatic capacity to help maintain brain health over many decades. Their ability to repress core pathological pathways common to many disorders – such as bioenergetic stress, oxidative stress, glutamate dyshomeostasis – suggests that targeting key regulators of these processes may have applicability across different diseases. Exploiting this knowledge to manipulate the astrocytic phenotype to boost their homeostatic support, and alter the trajectory in models of neurodegenerative disease, is an aim of the programme.
Generating hypotheses as to how the astrocytic phenotype is altered in vivo as a function of other cell types in the brain as well as the extracellular inflammatory milieu poses challenges due to the difficulty of modelling and interrogating the system. To aid this, the team has developed an in vitro system where neurogliovascular cell types from different species (e.g. rat, mouse, human) are maintained alone or in co-culture, followed by RNA-seq and in silico read separation according to species. This has led to the identification of gene networks and signals controlling astrocytic phenotype, validated in vivo, which the team plan to manipulate in vivo to test their effect on the neurodegenerative disease trajectory.
Downstream of dysfunctional astrocytes, one process that loss of homeostasis may converge on is excitotoxic synapse loss. Deficits in glutamate uptake capacity due to transporter hypoexpression or bioenergetic deficits associated with neurodegenerative disorders lead to its extracellular accumulation and chronic activation of NMDAR-mediated Ca2+ influx,. The group has already shown that excitotoxicity involves Mcu-mediated mitochondrial Ca2+ overload and also the coupling of the NMDAR to toxic NO production via PSD-95 and the C-terminal domain (CTD) specifically of GluN2B. They have the tools to genetically dissect the role of the GluN2B CTD and of Mcu in synapse loss in AD and other neurodegenerative disease models.
Main objectives and research goals:
- To determine how astrocytic properties are altered in health and in neurodegenerative disease.
- To assess the impact of modulating astrocyte properties.
- To assess the impact of neuron-modifying strategies for reducing excitatory synaptotoxicity.
- To create and use platforms to probing blood-brain-barrier function and inflammatory signal transduction across it.
Key publications
Vacancies
Lab members
- Dr Mosi Li (Postdoctoral Researcher)
- Dr Xin He (Bioinformatician)
- Dr Juraj Koudelka (Research Fellow/Imaging Lead)
- Dr Zoeb Jivaji (Clinical Consultant)
- Dr Jamie Loan (PhD Student)
- Kirsty Haddow (PhD Student)
- Kyle Wardlaw (PhD Student)
- Laoise Casserly (PhD Student)
- Katherine Ridley (PhD Student)
- Ruqi Zhang (PhD Student)
- Ya Yin Chang (PhD Student, joint with Prof Pato Opazo)
- Dr Owen Dando (Lead Bioinformatician)
- Dr Beverly Roberts (Centre Manager and Executive Assistant)
- Alexa Jury (Operations & Facilities Manager)
- Morag Laidlaw (Head of Research Management)
- Dr Deepali Vasoya (Bioinformatician)
- Lynsey Dunsmore (Colony Manager)
- Dr Alison Harris (Research Assistant)
- Mehreen Mohammad (Research Assistant)
- Abbey Lough (Lab Technician)
- Kirsty Haddow (Posdoctoral Researcher)
- Chloe Thimonier (Posdoctoral Researcher)
Collaborators











Lab funders
Thank you to all those who support the Hardingham Lab!