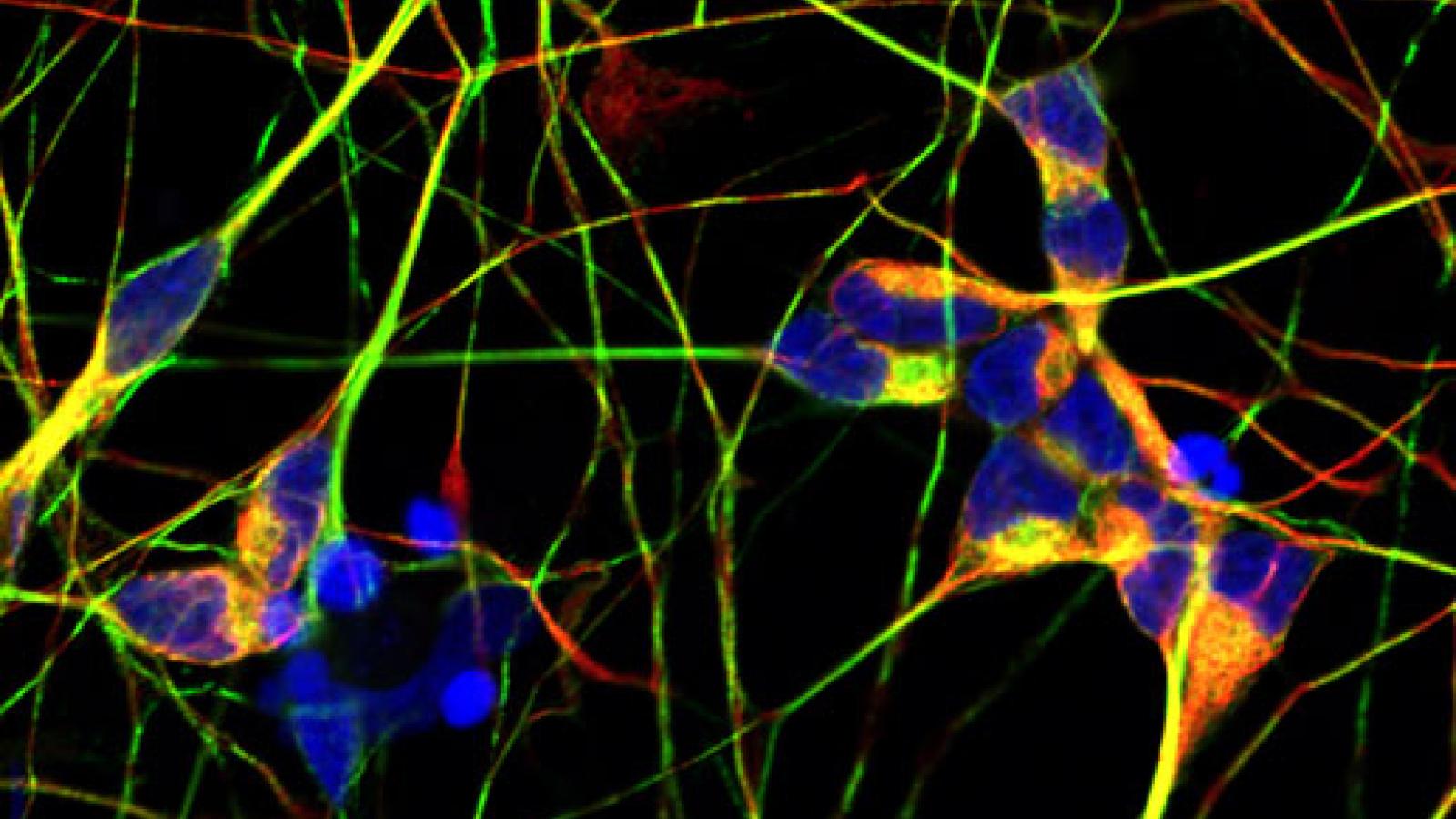
Tackling dementia: from genes to therapy
Frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS) are clinically distinct neurodegenerative diseases. But the discovery that they share a common cause – an expansion of a six-letter DNA sequence within the C9orf72 gene - is helping to revolutionize our understanding of the diseases.
Scientists are studying the biological impact of the faulty version of the C9orf72 gene to work out the mechanisms that lead to FTD and ALS. They theorise that the resulting protein may not function properly and/or be toxic to neurons.
The Isaacs Lab is investigating the underlying molecular mechanisms behind C9orf72-related FTD and ALS using a variety of experimental techniques and model systems. The team are also developing high-throughput screening approaches to identify genes and small molecules that modulate C9orf72 and other FTD/ALS genes. The ultimate goal is to develop innovative treatment strategies – such as novel gene therapies.
Lab news

Prof Adrian Isaacs
Prof Adrian Isaacs is a Group Leader at the UK DRI at UCL. Find out more about his career and expertise on his profile page.

Research summary
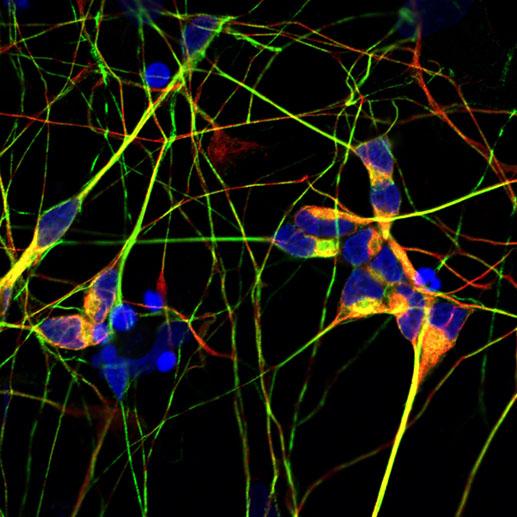
IPSC motor neurons. Credit: Isaacs Lab
Frontotemporal dementia and amyotrophic lateral sclerosis disease mechanisms
An intronic GGGGCC repeat expansion in C9orf72 is the most common genetic cause of both frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS). The repeats are translated by an unconventional mechanism termed repeat-associated non-ATG translation into five different dipeptide repeat proteins (DPRs). The team has previously shown that DPRs can be very damaging to neurons. They have now performed a large-scale modifier screen in fruit flies expressing GGGGCC repeats in adult neurons. They have identified several genes that improve the survival of these flies. These genes have not previously been implicated in C9orf72 FTD/ALS and so may represent completely novel pathways for rescuing C9orf72 repeat toxicity, and potentially other forms of neurodegeneration.
The team is now extending investigation of these genes using induced pluripotent stem cells (iPSCs) derived from people with the C9orf72 repeat expansion. They are also trying to decipher whether these genes can improve mouse models of C9orf72 repeat expansion and aim to determine if they are potential candidates for gene therapy.
The Isaacs Lab have also recently developed new 'knock-in' mouse models to investigate each of the individual DPRs. These knock-in mice provide a more accurate disease model because the level of the normal C9orf72 protein is also reduced - as is observed in human patients. There is increasing interest in whether loss of the normal function of C9orf72 contributes or sensitises to neurodegeneration. One reason for this is that C9orf72 is involved in endolysosomal function and is also expressed in immune cells. The team’s newly generated knock-in mice offer a unique opportunity to investigate these pathways and their contribution to disease.
Main objectives and research goals:
- Characterise new C9orf72 modifier genes in flies, iPSC-neurons and mouse models.
- Investigate new mechanisms by which the C9orf72 repeat expansion causes neurodegeneration.
- Investigate potential new gene therapy approaches for C9orf72 and related disorders.
- Utilise screening approaches to identify small molecules with therapeutic relevance for FTD/ALS.
Key publications
Vacancies
Lab members
- Dr Alex Cammack (Postdoctoral Researcher)
- Dr Georgina Starling (Postdoctoral Researcher)
- Dr Alla Mikheenko (Postdoctoral Researcher)
- Dr Paolo Marchi (Postdoctoral Researcher)
- Dr Mireia Carcolé (Senior Research Technician)
- Bailey Hewlett (Technician)
- Dr Cristina Marisol Castillo Bautista (Postdoctoral Researcher)
- Dr Martha Roberts (Postdoctoral Researcher)
- Alessia Fisher (Research Fellow)
- Ava Devaney (PhD Student)
- Stanislaw Majewski (PhD Student)
- Stephanie Taylor (PhD Student)
- David Orsulik (PhD Student)
- Dr Rubika Balendra (Clinical Student)
- Anastasia Garai (Student)
- Tommy Tang (Student)
- Finlay Dorsett (Student)
- Ekhlas Rahman (Senior Technician)
Collaborators












Lab funders
Thank you to all those who support the Isaacs Lab!