Investigating the role of immune cells in dementia
The McColl Lab are studying the role of specialised immune cells within the brain, called microglia, in neurodegenerative and cerebrovascular disease. Their goal is to gather new knowledge about which of their activities are harmful or helpful, and how this is controlled, paving the way to potential new treatments for dementia.
Microglia act as the brain’s sentry guards, seeking out potential threats. Once activated, they remove waste materials, dead cells or foreign invaders by engulfing them – and also enlist the help of other immune cells, leading to inflammation of the surrounding brain tissue. They also perform important “housekeeping” functions to maintain a healthy brain environment.
Excessive or misdirected microglial activity and inflammation, that may arise from certain states of reactive microglia, are a feature of many neurodegenerative diseases, including common forms of dementia such as Alzheimer’s disease (AD). But it remains unclear if the microglia’s disrupted function and inflammation is a root cause of disease or is a response to other triggers, such as the accumulation of abnormal proteins or death of neurons – other hallmarks of many neurodegenerative diseases. Malfunctioning blood vessels in around the brain may also be important triggers of microglial activity and the way microglia respond may also shape how vascular disease and events such as stroke may lead to dementia. Equally, it is emerging that insufficient or failed protective functions of microglia may also be involved in diseases leading to dementia. Understanding how the balance in microglial activity is controlled and how to optimise this therapeutically is an important target for therapy.
The McColl Lab are investigating how microglia sense and respond to tissue damage and stress signals, including from faulty brain blood vessels and abnormal protein aggregates, and how the involvement of tiny waste-recycling structures inside microglia, called lysosomes, may be involved in diseases leading to dementia. The team are also studying how microglia contribute to rare conditions known as ‘microgliopathies’, which are the most direct current evidence for malfunctioning microglia as causes of neurological disease – this will shed new insight into their biological functions and be instructive for how they may be involved in more common dementias. Finally, the team are also developing innovative new tools and techniques that will help advance the study of microglia and inflammation in neurodegenerative diseases.
Latest news
Dr Barry McColl
Dr Barry McColl is a Group Leader at the UK DRI at Edinburgh. Find out more about his career and expertise on his profile page.

Research summary
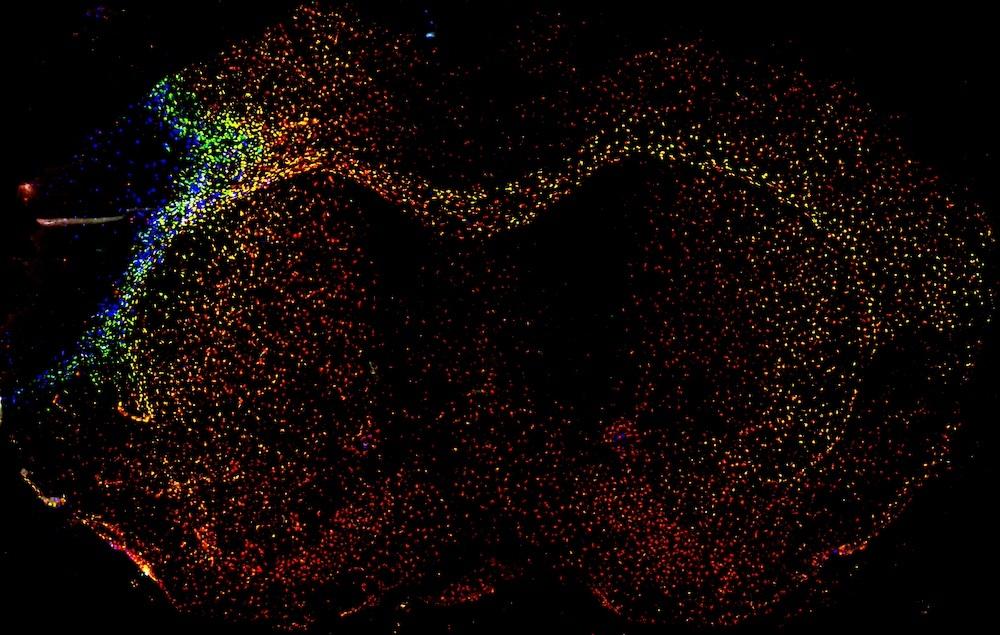
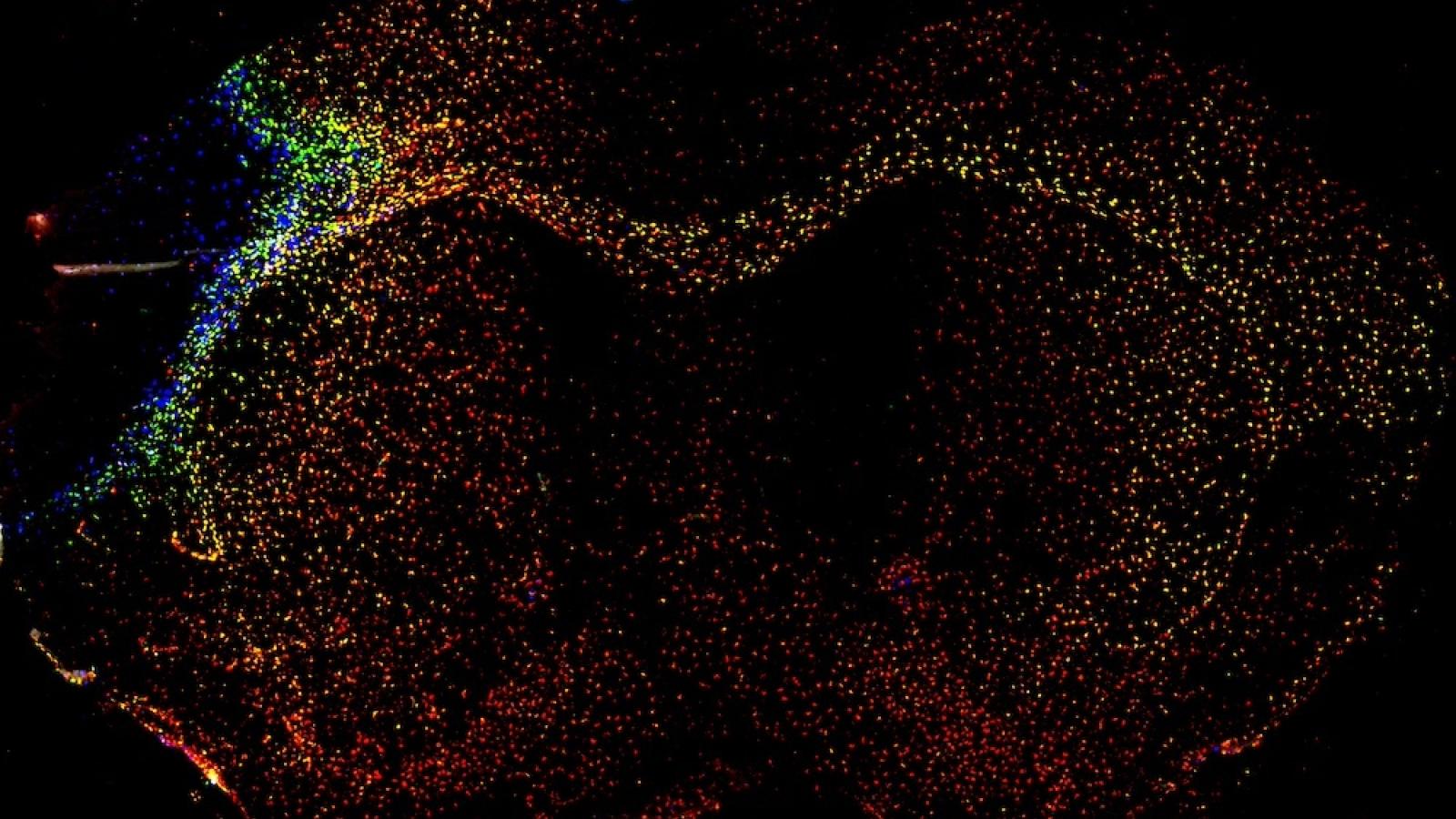
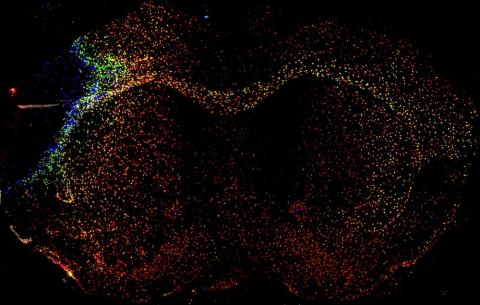
A recent paper from the McColl lab suggests that the brain's immune response to a stroke is widespread but organised based on brain structure. Credit: Jack Barrington
Microglial mechanisms influencing susceptibility and resilience to neurodegeneration
This UK DRI programme, led by Dr Barry McColl, contributes answers to some of the most pressing questions regarding the involvement of microglia and neuroinflammatory processes in neurodegenerative disease. The overall scientific goal is to identify key microglial mechanisms influencing resilience and susceptibility to dementia-causing disease, particularly arising from cerebrovascular disease, that could be manipulated for therapeutic intervention.
Microglia detect and respond to cellular and tissue distress and damage signals through an array of sensors. While considerable attention has been given to protein aggregates as triggers of microglial reactivity, a crucial knowledge gap is our poor understanding of what and how distress signals associated with cerebrovascular dysfunction and disease induce and/or propagate microglial reactive alterations. These signals may shape microglial responses early in disease course, but with potentially chronic consequences, so represent attractive targets for intervention. The lab is examining microglial sensing of stressors relevant to vascular dementia and Alzheimer’s disease, and the mechanisms regulating their responses.
Once reactivity is triggered, microglia can adopt a variety of altered molecular and functional states, some of which emerge contemporaneously and are co-located and some which are regionally variant. The combined actions of these distinct microglial subpopulations will influence their harmful and protective effects on disease and cognition. The McColl lab is investigating the ontogenetic and functional relationships among distinct reactive microglial subsets that emerge in disease states leading to dementia.
Among reactive microglial states identified recently, it is apparent that the altered gene expression patterns underpinning these states are heavily enriched for endolysosomal pathway genes. The team are conducting functional studies to determine roles of candidate microglial lysosomal pathways, and more broadly, are seeking to track microglial lysosomal changes during the course of neurodegenerative disease. Overall, the team aim to bring insight to mechanisms by which microglia sense, respond to and influence diseases leading to dementia, revealing targets to shape microglial responses favouring neurovascular and neuroimmune resilience to cognitive impairment
Main objectives and research goals:
- Determine triggers and transducing mechanisms of microglial reactivity in pathological states underlying dementia.
- Identify the ontogenetic, spatiotemporal, and functional relationships among reactive microglial states emerging during cerebrovascular and neurodegenerative disease, and their regulation.
- Define how cerebrovascular and neurodegenerative disease affects microglial lysosomal properties and functional consequences of perturbing microglial endolysosomal regulators.
Key publications
Vacancies
Lab members
- Dr Jack Barrington (Postdoctoral Researcher)
- Dr Gaia Brezzo (Postdoctoral Researcher)
- Dr Colin Crawford (Postdoctoral Researcher)
- Dr Mike Daniels (Postdoctoral Researcher)
- Dr Stefan Szymkowiak (Postdoctoral Researcher)
- Dana Straus (Research Assistant)
- Sarah Choi (Research Assistant)
- Veronica Villeri (PhD Student)
- Arlo Simmerman (PhD Student)
- Mila Redzic (PhD Student)
- Vanessa Fentor (Bioinformatician)

The McColl Lab based at the UK DRI at Edinburgh.
Collaborators






















Lab funders
Thank you to all those who support the McColl Lab!