Improving diagnosis, treatment and care for people living with dementia
Cellular and animal models have been vital to progressing our understanding of a multitude of diseases. However, in order to develop therapies for humans, there is no substitute for the real thing.
In this programme of work, the Fox Lab is working closely with clinical colleagues at the Dementia Research Centre (DRC), will be collecting clinical, imaging and neuropsychological data and biological samples from groups of people attending clinics, particularly those at risk of developing neurodegenerative conditions such as Alzheimer’s disease. The team will principally be investigating individuals which have genetic mutations predisposing them to these conditions, but also those that develop them sporadically.
By studying these individuals over a long period of time, it is possible to track and quantify the biological changes that occur even before symptoms start. This provides unique opportunities to gain a deeper understanding of what causes these diseases, to develop new tests for diagnosing dementia earlier, and to design and trial new drugs targeting each disease’s specific molecular signature.
Latest news


Prof Nick Fox
Prof Nick Fox is a Group Leader at the UK DRI at UCL. Find out more about his career and expertise on his profile page.

Research summary
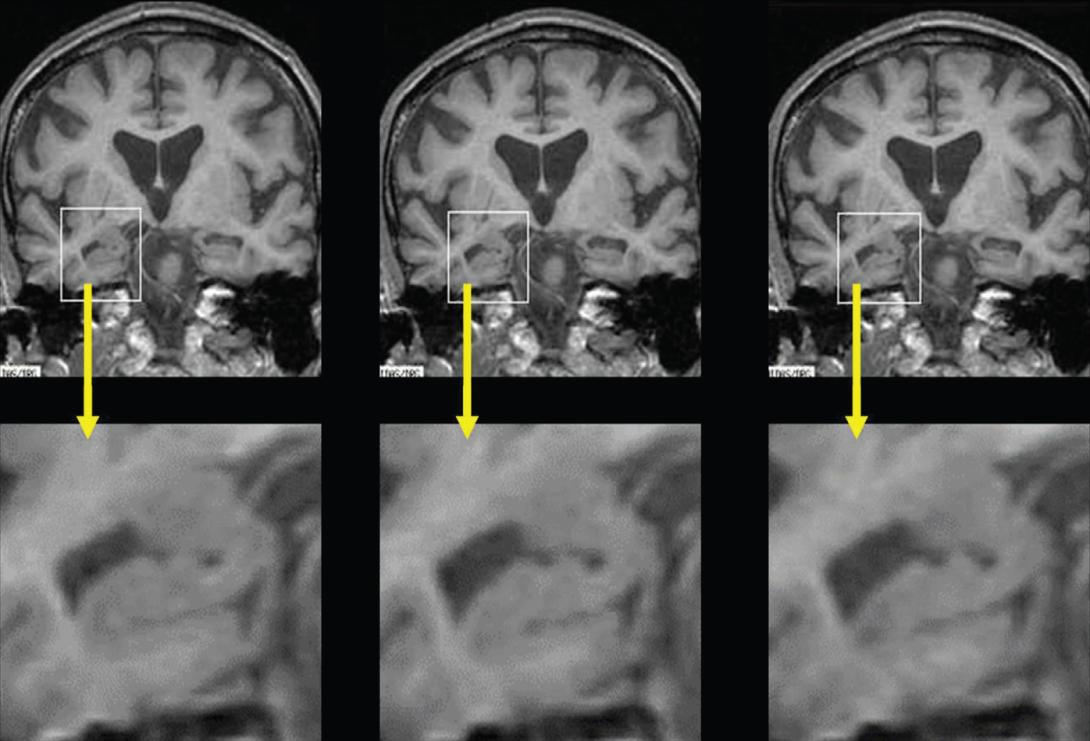
MRI scans showing the reduction of the hippocampus in Alzheimer's over a three year period.
Credit: Fox lab
From bench to clinic and back again
The capacity to carry out experimental medicine and find therapies is critically dependent on understanding disease in the human – there is no substitute for this. Therefore, access to clinical cohorts and associated materials is invaluable to the success of the UK DRI in its mission to translate basic research into therapeutics for dementia-associated conditions.
The Fox Lab and PIs at the DRC will support the effective integration of basic and clinical research by leveraging and building on ongoing studies and clinical expertise available at the DRC at UCL which hosts a number of longitudinal cohort studies including: (a) at-risk genetic populations of familial Alzheimer’s disease and frontotemporal dementia (FTD); (b) sporadic FTD, AD, and Lewy body dementias; and (c) biomarker (positive and negative) ageing birth cohorts where life course influences and genetics can be accounted for.
Specifically, this programme will: streamline and centralise the collection and storage of materials – DNA, RNA, serum, plasma, CSF, fibroblast- and blood-derived hiPSCs and brain tissue – from these cohorts, providing a vital resource to support the ground-breaking work of other groups at the DRI; and facilitate the identification of patients with familial forms of dementia throughout the UK, recruitment to research studies, and provide support to patients, family members and at-risk individuals.
The clinical cohorts available – and particularly individuals at risk of familial dementia – will be ideally placed to underpin first-in-human therapeutic studies, vital for translating basic research once viable drug targets are identified.
Main objectives and research goals:
- Assess and offer research to every UK family with familial dementia, establishing a national centre, providing support to patients through specialist clinicians and support groups, and offering therapeutic trials at all stages of disease.
- Establish a comprehensive collection of UK dementia samples: from blood & CSF to brain donation.
- Establish novel dynamic markers at UCL, e.g. SILK (stable isotope labelling kinetics).
- Use ex-vivo (e.g. iPSC) and in-vivo (imaging and biomarker) studies, to better define the relationships between mutation and effect on clinical manifestation and biomarkers.
- Support multi-centre, multi-disciplinary familial dementia collaborations.
- Establish a research programme to develop and test gene-based therapies in neurodegeneration.
- Run at least one pilot therapeutic trial in genetic forms of dementia.
- Conduct research into improving recruitment, acceptability and retention of clinical trials – including support for families and carers.
Key publications
Vacancies
Lab members
- Emily Abel (Research Technician- joint with Prof Henrik Zetterberg)
- Dr Moneeb Nasir (Postdoctoral Researcher)
- Dr Chris Hardy (Postdoctoral Researcher)
- Dr David Cash (Professorial Research Fellow)
- Dr Ross Paterson (Senior Clinical Researcher and Consultant Neurologist)
- Dr Natalie Ryan (Senior Clinical Researcher and Consultant Neurologist)
- Dr Miguel Grilo (Clinical Researcher)
- Dr Xin Zhang (Clinical Student)
- Helen Rice (Research Nurse)
- Llwyd Prosser (Senior Imaging Technician)
- Erinna Bowman (Study Coordinator)
- Christine Chow (Neurogenetic Therapies Programme Manager)
- Dr Philip Weston (Emerging Leader and Consultant Neurologist)
- Suzie Barker (Project Officer)
- Millie Beament (Researcher Assistant)
Collaborators







Lab funders
Thank you to all those who support the Fox Lab!