In neurodegenerative conditions such as Alzheimer’s, changes in the brain begin years, perhaps decades, before symptoms appear. Biomarkers – short for “biological markers” – are signals that these subtle biological changes are happening. Detecting them will give us the best chance of intervening early and slowing down, or perhaps even preventing, the onset of symptoms.
With new treatments on the horizon, we urgently need accurate, scalable diagnostic tools to identify people who could benefit. At present, only 2% of people with dementia symptoms receive a specialist test to confirm the underlying disease, e.g. Alzheimer’s. This is partly because current diagnostic methods are invasive and/or expensive and resource-intensive for the NHS to provide. UK DRI researchers are studying a range of biomarkers that could be used to develop new, scalable diagnostic tests – including blood tests.
Biomarkers are also critical for testing emerging new treatments. Detecting early signs of disease will help scientists identify suitable participants for clinical trials, and biomarkers can also measure participants’ progress to determine whether and how well the treatment is working. This is particularly needed in neurodegeneration, a field in which clinical research has historically been challenging.
A deeper dive
UK DRI researchers are working to find and validate different types of biomarkers across a range of neurodegenerative diseases.
Fluid-based biomarkers
Representing the global gold-standard, the UK DRI Biomarker Factory is a high-performance analytical platform for fluid biomarker development, validation and measurement. Led by world expert Prof Henrik Zetterberg and co-led by Dr Amanda Heslegrave, it is the most experienced academic lab in the UK, and the first to have the latest Alamar NULISA technology.
The UK DRI Biomarker Factory conducts world-leading research across a range of blood (plasma/serum) and cerebrospinal fluid (CSF) biomarkers, including pioneering at-home blood tests to predict and monitor dementia. As part of the Blood Biomarker Challenge, we are supporting two teams to validate novel blood tests for NHS use. For more information on the Biomarker Factory, visit the dedicated page here.
In motor neuron disease/ALS, Prof Adrian Isaacs has developed a biomarker test sensitive enough to monitor levels of a specific protein in spinal fluid, which signals a faulty C9 gene – a mutation responsible for approximately 40% of familial cases. The new test is very accurate, and is already being used in a clinical trial to test a new MND/ALS therapy.
In Parkinson’s, Dr Tim Bartels has discovered that a specific structural form of the protein α-synuclein can be detected in blood and harnessed as a fluid biomarker for the early detection of disease.

UK DRI Biomarker Factory aims to transform the early detection, diagnosis and monitoring of dementia, by facilitating the development of fluid biomarkers for research and the clinic
Dr Shlomi Haar is developing motor digital biomarkers which can account for daily life activities in the real-world and fluctuations in posture, mobility, frailty, and movement structure
Digital biomarkers
Biological changes can also be detected by digital technology. Dr Cynthia Sandor led a study that found smart watch data can detect Parkinson’s up to seven years before hallmark symptoms appear. In our Centre for Care Research & Technology, Prof David Sharp is exploring whether data from home sensors, including a sleep mat, can indicate an individual’s neurodegenerative disease status and progression towards dementia. , and Dr Shlomi Haar is measuring body movement to better understand and track disease progression.
Functional biomarkers
UK DRI researchers are also studying subtle cognitive changes that signal the onset of Alzheimer’s. Dr Marc Aurel Busche is harnessing whole-brain magnetoencephalography (MEG) and machine learning to explore how different forms of neural replay are affected. Prof Karen Duff is examining cellular changes in mice and early behavioural changes in humans at risk, to understand how Alzheimer’s pathology at the cellular level may result in early cognitive changes.
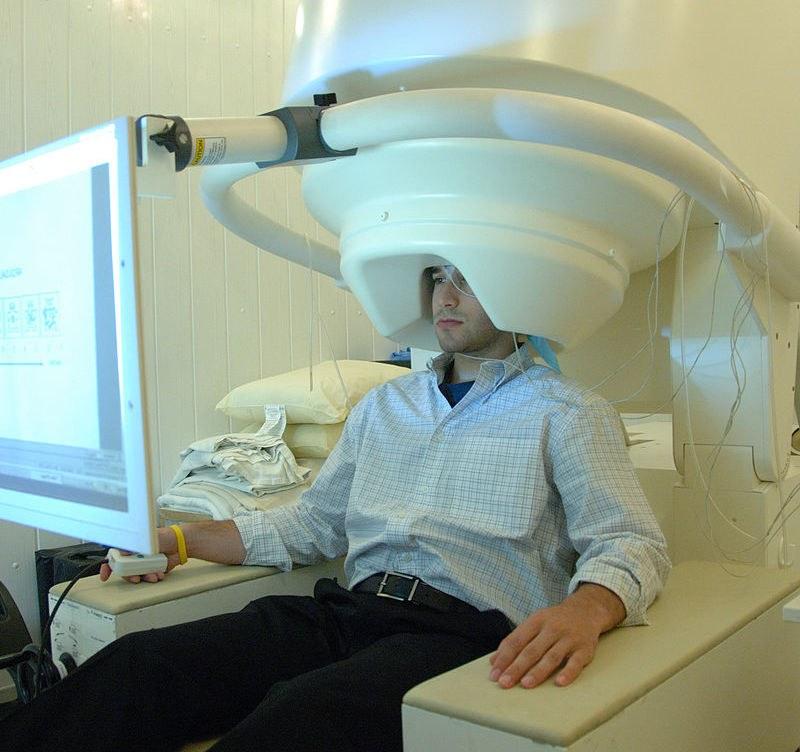
Dr Marc Busche and collaborators will harness whole-brain magnetoencephalography (MEG) and machine learning to explore how different forms of neural replay are affected during disease progression. Credit: National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services
Genetic risk
Our genes impact our risk of developing certain conditions including those that lead to dementia. Prof Julie Williams conducts pioneering research into how our genes influence our risk of Alzheimer’s, including working on the 92 risk genes now identified. Prof Valentina Escott-Price, an expert in “big data”, uses large datasets to examine total genetic risk in order to determine a polygenic risk score for individuals.