Key details
Understanding how brain connections are regulated in Alzheimer's
The adult brain is not hard wired, but can reorganise and learn about changes in the world around us. The remarkable computational power of the brain is in part due to its unique structure: billions of individual brain cells are connected to form circuits by tiny contact points called synapses. Synapses facilitate the flow of electro-chemical signalling through brain circuits, and this is widely believed to support memory, learning and perception. In Alzheimer's disease and ageing, synapses become unhealthy and then are lost. The exact process by which this happens remains unclear. In much the same way as your body regulates temperature to prevent extreme levels, synapses also regulate the electrical activity of the brain. This process is called synaptic homeostasis and is thought to be critical for preventing extreme levels of hyperactive brain cell activity which have been reported in Alzheimer's. Synaptic homeostasis is impaired in both the ageing brain and the very early stages of Alzheimer's, and this impairs memory.
The goal of the Barnes Lab is to understand what causes impaired synaptic homeostasis in ageing and Alzheimer's and use this information to develop approaches that can improve synaptic health. To date, the team have found common molecules involved in synaptic homeostasis are dysregulated in both ageing and Alzheimer's, and have developed drugs that can boost synaptic homeostasis, which can also improve memory.
Neurons firing. Credit: Barnes Lab
Latest news


Dr Samuel Barnes
Dr Samuel Barnes is a Group Leader and Interim Deputy Director at the UK DRI at Imperial. Find out more about his career and expertise on his profile page.

Research summary
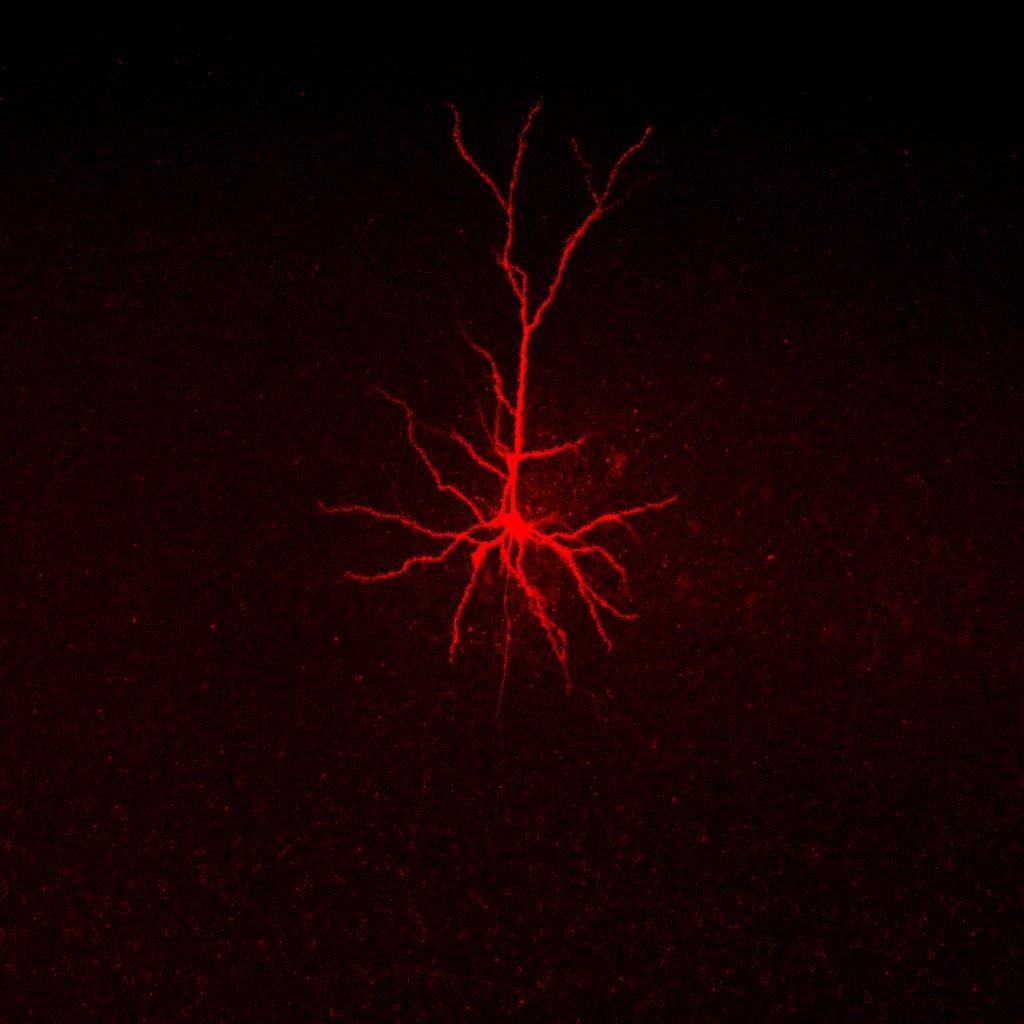
Confocal image of an excitatory pyramidal neuron filled with internal solution containing red dye. Credit: Carola Radulescu & Nazanin Doostdar
Micro-circuit homeostasis in ageing and early-stage Alzheimer's
Dr Barnes's research focuses on homeostatic plasticity mechanisms that regulate neural firing-rate activity within a stable dynamic range, preventing prolonged periods of hyper- or hypo-activity. The lab hypothesise that homeostatic control is neuroprotective but may fail in ageing and the early stages of neurodegeneration, leading to pathophysiological neural-circuit activity. To test this hypothesis, Sam's team uses a combination of electrophysiology, 2-Photon calcium imaging and 1-Photon brain-wide imaging. They combines these approaches with molecular measures such as transcriptomics and spatial proteomics to probe the mechanisms involved in homeostatic control, as well as behavioural testing to measure the consequences of destabilized neural-circuit activity.
Main research objectives:
- To determine the role of ageing in destablising homeostatic plasticity mechanisms and the consequences for age-related cognitive decline.
- To investigate how early-stage amyloidosis impacts synaptic homeostatic control and cognition.
- To develop and test, pharmacological, genetic and bioelectronic interventions to promote synaptic homeostasis in ageing and early-stages of amyloidosis.
Key publications
Vacancies
Lab members
- Dr Carola Radulescu (Senior Researcher)
- Dr Maria Sabina Cerullo (Postdoctoral Researcher)
- Dr Nazanin Doostdar (Postdoctoral Researcher)
- Dr Kjara Pilch (Postdoctoral Researcher)
- Dr Leire Melgosa (Postdoctoral Researcher)
- Xingjian Wang (PhD Student)
- Fran Chaloner (Research Assistant)
- Isabella Russell-Smith (PhD Student)
- Eline Stas (PhD Student)
Collaborators









Lab funders
Thank you to all those who support the Barnes Lab!





