Info
Characterising protein clumps in the brain
Neurodegenerative diseases are a major health problem for mankind and are due to the slow clumping of proteins in the brain as we age. These small protein clumps initiate and drive the spreading of the disease through the brain. Unfortunately, these clumps are so low in concentration in human brains or fluids that are invisible to most methods, making it impossible to detect or characterise them.
To address this problem the Klenerman Lab has developed a set of new methods that can detect and characterise individual protein clumps that work on human samples. The group seeks to apply these methods to samples of human brain, spinal fluid and blood at different stages of disease available from my clinical colleagues. This will allow the lab to determine which clumps form initially and how they damage the brain, how these clumps change as the disease develops and how these clumps differ between the different diseases.
This is fundamental new information about what happens in the human disease which will allow one to know which clumps to target for therapies. These methods also have great potential for the early diagnosis of disease, especially Parkinson’s disease for which no such diagnostic method exists.
Latest news

Prof David Klenerman
Prof David Klenerman FMedSci FRS is a Group Leader at the UK DRI at Cambridge. Find out more about his career and expertise on his profile page.

Research summary
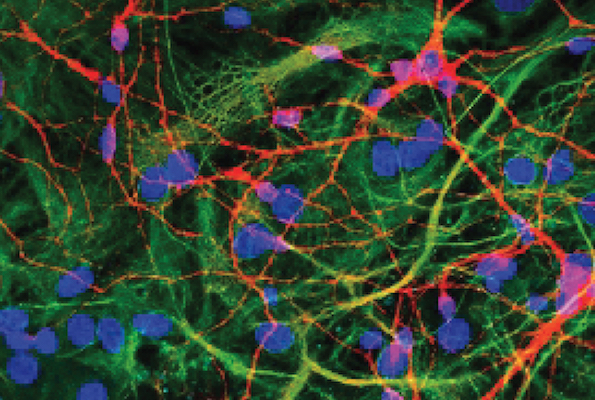
Klenerman Lab's culture.
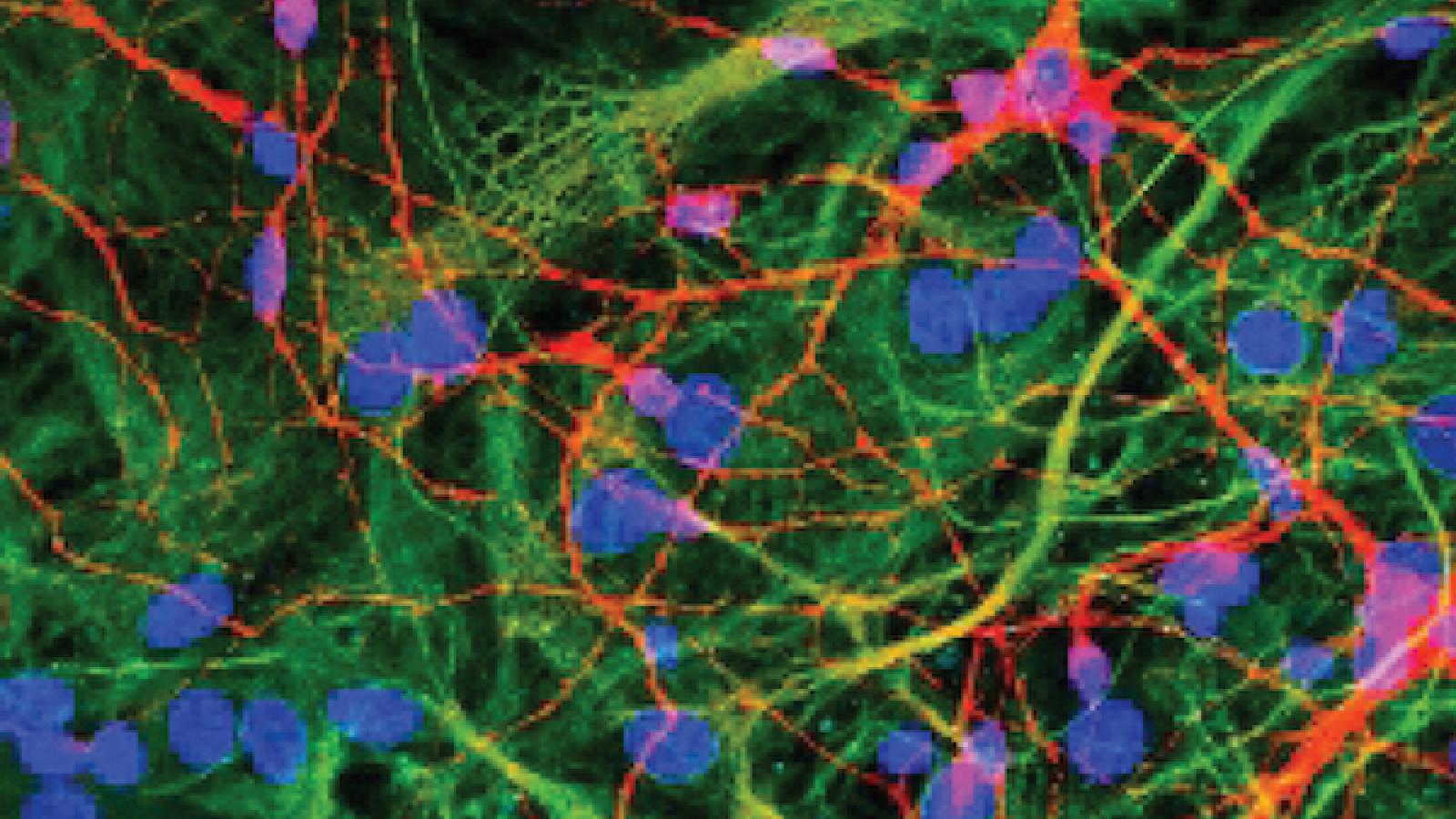
Soluble protein aggregates of tau that form at the onset of the disease
Soluble protein aggregates of tau, beta amyloid and α-synuclein, with a range of different sizes and structures, are formed during the initiation and development of neurodegenerative disease and are toxic to cells by a variety of different mechanisms. However, currently there is a lack of methods to detect and characterise these soluble aggregates, especially in human-derived samples, due to their low concentration and heterogeneity.
To address this issue, the Klenerman Lab has developed a suite of new sensitive and selective biophysical methods. These methods can measure the number, size down to 20 nm, proteinase K resistance, composition and key post-translational modifications of protein aggregates in serum, cerebrospinal fluid (CSF) and extracted from post-mortem brain and which of these aggregates are bound by APOE. These methods can also measure the capability of aggregates to cause permeabilisation of a lipid membrane and inflammation.
The team aims to use these methods to characterise the aggregates found in control and diseased human brain samples, in different regions of the brain and at different stages of disease, and the aggregates present in the CSF and serum of patients and controls. They will use samples of control, Alzheimer’s disease (AD), Parkinson’s disease (PD) and tauopathy disease brains, available in the Cambridge brain bank, and serum or plasma of patients to determine which aggregates form first, how they change as disease progresses and how they differ between the different diseases. Moreover, changes in synaptic function is one of the earliest signs of disease, so the Klenerman Lab seeks to study the changes in the structure of synapses in different brain regions by performing super-resolution imaging of synaptosomes, produced from post-mortem human brain, and characterising the protein aggregates present.
The team's key objectives are to:
- Characterise the soluble aggregates present in control patient, serum and post-mortem brain.
- Characterise the soluble aggregates present in AD, PD and tauopathy disease serum and post-mortem brain.
- Characterise the synaptosomes and the aggregates present in synaptosomes of control, AD, PD and tauopathy disease post-mortem brain.
Overall, this work will characterise the aggregates that form in humans during the initiation and development of disease at unprecedented levels of detail, providing new targets for therapies and new early disease diagnosis.
Vacancies
Lab members
- Dr Shekhar Kedia (Research Associate)
- Dr Evgeniia Lobanova (Research Associate)
- Dr Rohan Ranasinghe (Research Associate)
- Dr Georg Meisl (Research Associate)
- Dr Yu Zhang (Research Associate)
- Dr Arpan Dey (Research Associate)
- Dr Debasis Banik (Research Associate)
- Dr Matthew Cotton (Postdoctoral Researcher)
- Tomi Akingbade (PhD Student)
- Dorothea Boeken (PhD Student)
- Elizabeth English (PhD Student)
- Prasanna Suresh (PhD Student)
- Beatrix Huissoon (PhD Student)
- Asher Dworkin (PhD Student)
- Kelly Xiong (PhD Student)
- Yuhao Cui (PhD Student)
- Eva Wong (PhD Student)
- Daniel Heraghty (PhD Student)
- Florence Layburn (PhD Student)
- Shih-Huan Huang (PhD Student)
- Jae Eun Kim (Janice) (PhD Student)
- Kim Jae Eun (PhD Student)
- Shaan Baig (PhD Student)
- Nicola Yeung (PhD Student)
- Anna Conti (PhD Student)
- Nadia Karimpour (Lab manager)
- Cherida Zhang (Research Assistant)
Key publications
Collaborators



















Lab funders
Thank you to all those who fund the Klenerman Lab!