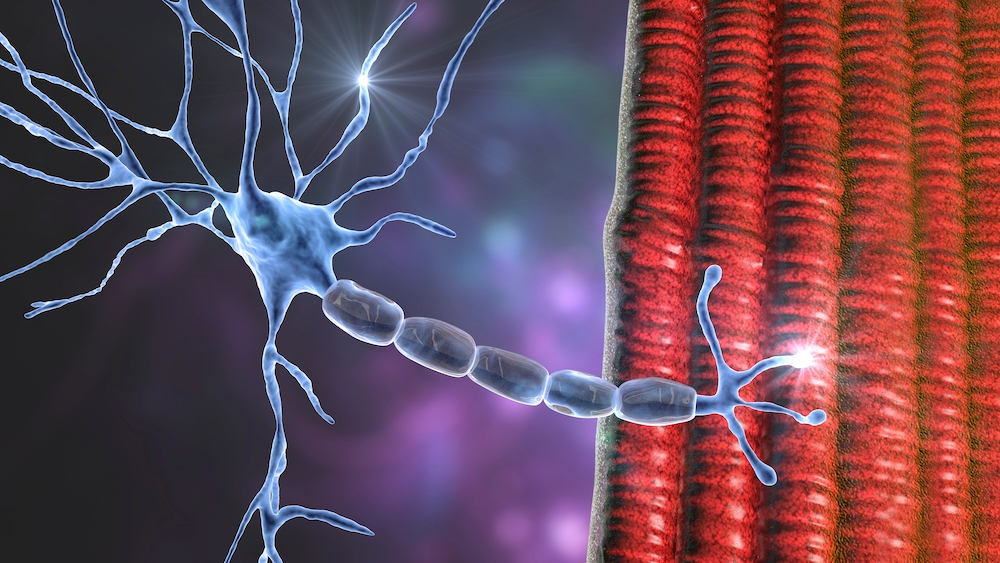
Motor neuron disease (MND)/ amyotrophic lateral sclerosis (ALS) stands among the most devastating neurological conditions – progressive, currently incurable, and ultimately fatal. At the UK DRI, our commitment to improving the lives of people living with MND/ALS has been unwavering since our inception, with our many teams across the UK driving breakthroughs from fundamental discoveries to clinical trials.
Through pioneering research spanning laboratory insights to innovative therapeutic approaches, we continue to drive advances to understand this challenging condition. Our world-class researchers, including Dr Marc-David Ruepp, Dr Sarah Mizielinska and Prof Suvankar Pal, are spearheading multifaceted efforts that range from unravelling MND/ALS's complex biology to accelerating promising treatments through groundbreaking trial designs. Their work exemplifies our institute's comprehensive approach: transforming scientific discoveries into tangible hope for people affected by MND/ALS.
Steve Barrett OBE, who lives with MND and is a participant on the clinical trial MND-SMART, said:
“The solution to cracking the MND code will come and after experiencing, and feeling, the expertise of those working on finding those solutions, I am more confident than I have ever been that positive change is only over the horizon; we will see it soon.”
Why are there so few treatment options for people affected by MND?
There is an urgent unmet need to improve outcomes for people affected by MND/ALS, estimated to affect 1 in 300 people in their lifetime. The average life expectancy for the condition, which causes progressive muscle weakness due to the degeneration of motor neurons in the brain and spinal cord, is just two to three years. Sadly, there is only one drug licensed for use for the condition in the UK: riluzole was approved in 1995 and has a moderate effect, prolonging life expectancy by just a few months.
“It’s not through lack of trying,” Prof Suvankar Pal says, of the scarcity of therapies available. “There have been over 125 MND clinical trials over the past decade, but these have failed for a number of different reasons.”
Clinical trials have had too few participants, he explains, with too short a follow up time, and imprecise measures of treatment effectiveness. In earlier stage research, limitations with animal and cell models have created added challenges.
“Because the underlying biology of the disease is so complex,” Prof Pal explains, “we don’t have a perfect model to take things forward from the lab into the clinic.”

UK DRI scientists continue to drive advances in MND/ALS research.
How are UK DRI labs charging a ‘pivotal moment’ in MND research?
Major advances in our genetic and molecular understanding of MND/ALS over the last two decades, combined with the emergence of powerful tools to study the disease, offer hope. The UK DRI has 11 dedicated MND/ALS research programmes, focused on uncovering the biological mechanisms, developing novel diagnostic tools, identifying targets for new drugs, and accelerating clinical trials, already making significant progress.
Across neurodegenerative conditions, biomarkers are bringing huge optimism. Prof Adrian Isaacs (UK DRI at UCL) has developed a biomarker test, a measure or ‘flag’ that in this case indicates the presence of a faulty protein, to monitor the success of gene therapies for MND/ALS and frontotemporal dementia (FTD). The promising test has been used as part of a clinical trial.
Researchers at the UK DRI are hopeful that growing understanding of the condition is poised to translate to tangible benefits in the clinic.
“We’re at a pivotal moment in MND research,” Dr Sarah Mizielinska says. “We have a high-level understanding of the key cellular pathways involved, and we’ve started to get to the point where gene therapies are being introduced.”
One drug, tofersen, has been licensed in the US for people with MND/ALS who have mutations in a gene called SOD1, which is around 2% of those affected by the condition.
“This particular gene therapy for this rare form of MND isn’t a cure,” Dr Marc-David Ruepp says, “but it shows that if you understand a disease mechanism, and design a drug well, disease can be slowed. And that is transformative because it proves it is possible to alter the disease course of MND.”
MND is also proving to be a pathfinder for the wider field as pathology that occurs in MND/ALS is found in people with other neurodegenerative conditions, including FTD and a proportion of Alzheimer’s. This is enabling rich collaboration between researchers in different disease areas including Dr Sarah Marzi, an epigeneticist investigating gene regulation and expression in Alzheimer’s and Parkinson’s, who is now applying this knowledge to MND/ALS.
Dr Sarah Mizielinska explains how her team examines the tiny cellular transport systems that keep our neurons healthy - tracking individual molecules to understand why they break down in motor neuron disease and frontotemporal dementia.
How is MND paving the way in clinical trial design?
Alongside breakthroughs in our understanding of MND/ALS, an important piece of the puzzle is the revolution in clinical trials. MND-SMART (Systematic Multi-arm Adaptive Randomised Trial) is an innovative type of trial which evaluates multiple treatments simultaneously in a multi-arm, multi-stage adaptive platform. In 2024, the first results from the trial were published. Although neither of the first two drugs tested improved outcomes, the unique design and scale of the trial enabled results to be reached in three years, less than half the time it would have otherwise taken.
This platform not only speeds up the time it takes to test new drugs, but enables wider participation in research. The trial is spearheaded by Prof Suvankar Pal, and UK DRI Director Prof Siddharthan Chandran, at the Euan MacDonald Centre at the University of Edinburgh. Prof Pal says there has also been a culture shift in recent years, with more and more people keen to get involved in research.
Steven Morris, an MND-SMART participant, said: “Nothing is worse than living with MND. We will participate in trials of any drugs that may work to slow, stop, and reverse this disease – regardless of potential side effects.”
“The plan to introduce new drugs for testing in MND SMART opens up a new avenue of hope for all of us," he added. "Unless we try new things we are stuck in the same place.”
Before the new trial was set up four years ago, less than 5% of people with MND/ALS participated in drug trials, and these were based at a handful of centres of excellence, in urban areas of the country – limiting access depending on where participants were situated. Through MND-SMART, investigators collaborate with trial delivery sites to create pockets of expertise across the UK, and are nearing the 1000 participant milestone.
“MND-SMART has democratised access to clinical trials in the UK,” Prof Pal explains. “We have 23 sites across all four UK nations, we’ve built up infrastructure and training, drawing on expertise from local clinicians and nurses.”

MND-SMART has democratised access to MND clinical trials in the UK. Credit: Maverick Photo Agency
How is UK DRI connecting labs and clinics for MND?
To accelerate progress in MND/ALS research, the UK DRI MND research community have recognised that creating strong links between discovery and clinical research is key.
“One of the strengths we have within the community, is that our expertise spans from cellular-molecular discovery to clinical trials, and indeed this is bi-directional as we enhance our human focused research,” Dr Mizielinska says. “That’s pretty remarkable.”
From fundamental MND/ALS researchers like Dr Mizielinska and Dr Ruepp, to clinicians like Prof Chandran and Prof Chris Shaw, UK DRI researchers have a wide breadth of expertise.
Supported by the UK DRI’s gene therapy platform, Prof Shaw co-founded the UK DRI spinout company AviadoBio in 2021, focused on developing gene therapies for MND/ALS and frontotemporal dementia. A multi-million pound partnership with LifeArc is pushing ahead some exciting MND/ALS projects, including a drug repurposing project led by Prof Chandran, which additionally feeds into MND-SMART, helping to speed up the identification of target drugs to go into testing in the trial. In another project, UK DRI researchers are collecting blood samples from MND-SMART participants, to measure the levels of biomarkers related to their condition, and how they change over time. The UK DRI continues to invest in early-stage MND/ALS research, through its pilot studies programme.
The researchers are aligning their ways of working, and streamlining processes so that new ideas and information can rapidly be implemented.
Dr Ruepp explains: “For example, if we find something interesting in our cutting-edge models, we can then quickly test it in patient samples and confirm its direct relevance to disease. And in reverse, if something comes up in the MND-SMART trial, we can run it through our disease models, to get to the mechanism and understand the underlying cause. Our research is highly translational.”
This uniquely bi-directional pipeline is what makes the work so exciting, with hugely promising opportunities for new treatments. The UK DRI team are keen to build on this, through the relationships and partnerships they have built with external researchers and initiatives – and through forming new ones.
“We want to build on the strength of the UK DRI and our external partners to enhance our pipeline,” Dr Ruepp adds. “We don’t want to be siloed, this is a conduit we can build to link together all the great work already happening.”
Talking to the three scientists, it is clear they are passionate about a common goal – to improve the outlook for people living with this devastating condition.
“The goal is to get something for the patients. We need information, we need to find a cure,” Dr Ruepp says. “The patients are the mission.”
Links




UK DRI labs working on MND/ALS



Motor neuron disease/ALS
Learn more about MND/ALS and how researchers at the UK DRI are tackling the condition, from discovering the causes, through to developing tools and treatments, and delivering pioneering clinical trials.